In April 2016, optometry lost a giant when the author of the seminal work Primary Care of the Posterior Segment, Larry Alexander, OD, died. In addition to being an optometric physician, author and educator at the University of Alabama Birmingham School of Optometry, Dr. Alexander was a past president of the Optometric Retina Society (ORS). That group has chosen to honor his legacy by accepting case reports from optometric residents across the country relating to vitreoretinal disease. As selected by the board of the ORS, this case won the third annual Larry Alexander Resident Case Report Contest.
A patient who presents with congenital optic disc pit (CODP) is at risk for developing optic disc pit maculopathy (ODPM). Many published studies have explored surgical techniques for treating this condition. Despite our current understanding of this complication, no consensus regarding the optimal treatment for ODPM exists. In addition, the literature shows no clear consensus on the mechanism of pathogenesis or the origin of the subretinal fluid, making it more difficult to determine the optimal surgical technique for the treatment of such a complex condition.
Here, we review the presentation, diagnosis and surgical options for a patient who presented with this condition.
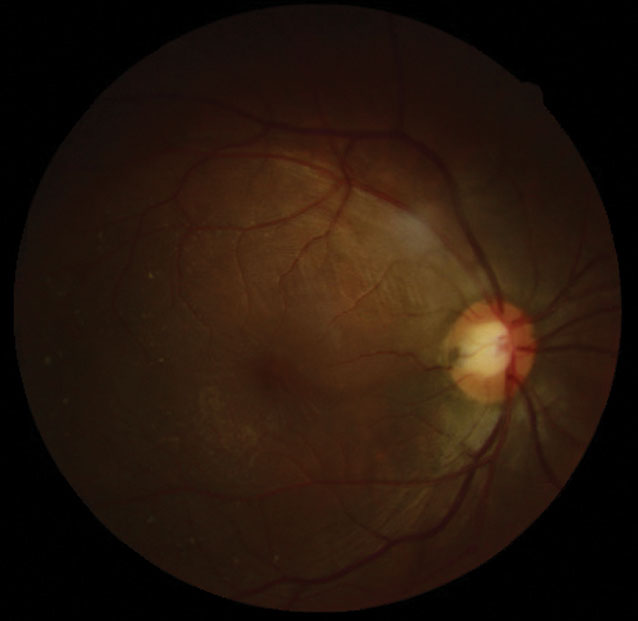 |
| The patient presented with a temporal ODP and an elevated macula with surrounding radiating stellate pattern. Click image to enlarge. |
Background
CODP presents as small, round, hypopigmented excavated defects usually within the temporal (or inferior temporal) portion of the optic disc.1 They are grayish white and typically unilateral (although 10% to 15% of cases are bilaterally).1,2 CODP defects vary in both depth and size. Their average diameter is 500µm and can be up to 0.16mm deep. CODP occurs in one out of 11,000 patients.1 No sex nor genetic predilections are associated with this condition.3,4 Due to their congenital nature, they are often diagnosed on the patient’s initial eye exam.3 Though visual acuity (VA) is rarely affected, field loss is possible. If field loss is present, the defect corresponds with a paracentral arcuate scotoma or an enlarged blind spot or both.5,6
Any patient who presents with a CODP is at risk for developing ODPM. This complication presents as a serous-neurosensory detachment in conjunction with the presence of an optic disc pit. Occasionally, serous maculopathy presents together with a retinoschisis. ODPM can occur in 52% of patients with CODPs. Patients with temporally located pits are at the greatest risk for developing serous maculopathy.5
Typically, maculopathy occurs in patients between 30 and 40 years old.6-8 In the presence of this complication, VA is usually reduced to 20/70 or worse.7
Though spontaneous resolution occurs in 25% of patients, the majority of cases have a poor visual prognosis, with gradually decreasing vision, leading to a final VA of 20/200 or worse.7,9 The cause of ODPM remains unclear.7
Surgical intervention has proven to be extremely valuable in managing these patients; yet, due to the rarity of such a unique condition, few cases exist published in academic literature.
Despite our current understanding of this condition, researchers have reached no consensus regarding the optimal treatment.6
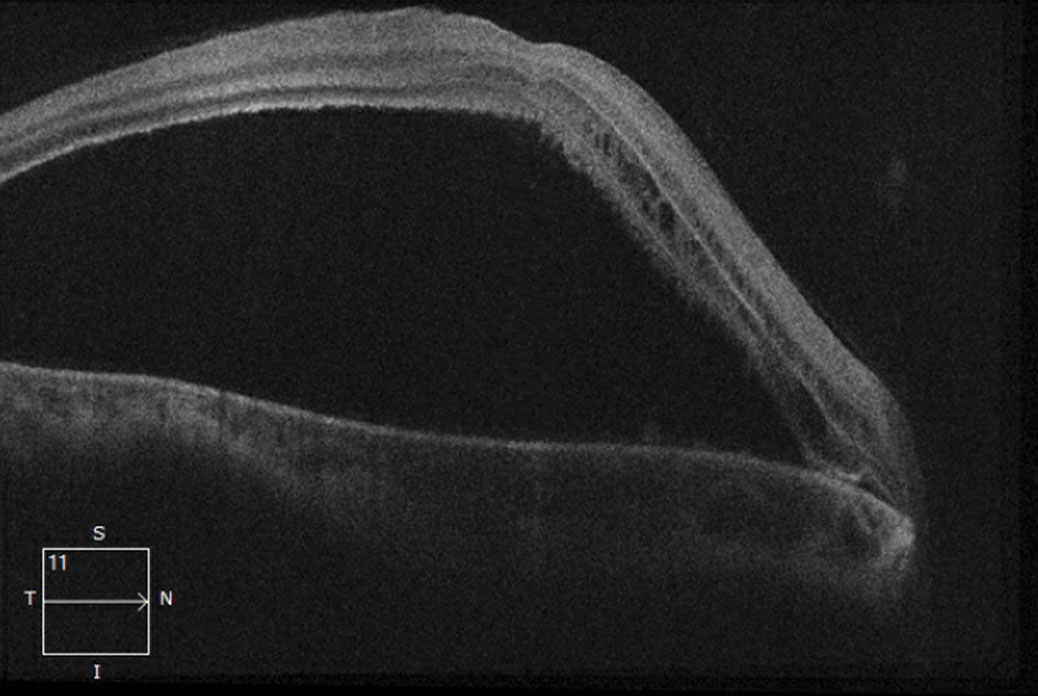 |
| This 21-line macular OCT shows, in our patient’s right eye, a non-rhegmatogenous serous retinal detachment. Click image to enlarge. |
Not Rare in Your Chair
A 29-year-old African-American female presented with a chief complaint of constant, painless, longstanding unilateral vision loss. She reported that her vision gradually began to decrease eight months prior to her exam and has remained poor since that time. She denied any ocular trauma, but her history was remarkable for compound myopic astigmatism in both eyes, and her medical history was remarkable for asthma.
Her best-corrected VA was 20/400 OD and 20/20 OS. When retinoscopy findings were compared with the patient’s current spectacle prescription, a significant hyperopic shift was noted in the right eye along with a dim light reflex, which was greater in the right than the left eye.
Dilated fundus evaluation and posterior segment photos of the right eye show a deeply pigmented circular hole in the temporal neuroretinal rim of the optic nerve. The macula and the temporal juxtapapillary retina appeared elevated, and a radiating stellate pattern surrounded the macula.
Optical coherence tomography (OCT) of the right eye showed an accumulation of serous fluid separating the sensory retina and the retinal pigment epithelium (RPE). Cystic pockets were noted in the outer retinal layers (nasal to the fovea) on OCT.
Differentials
The differential diagnosis for a serous retinal detachment is dependent upon the underlying etiology. The four etiologies are neoplastic, inflammatory, vascular and congenital. Neoplastic causes include choroidal melanoma, choroidal hemangioma and choroidal metastatic lesions, while inflammatory causes include Vogt-Koyanagi-Harada disease, posterior scleritis and sympathetic ophthalmia. Common vascular etiologies include choroidal neovascularization, Coats’ disease, malignant hypertension and familial exudative vitreoretinopathy. Congenital lesions include CODP, morning glory disc anomalies and choroidal coloboma.10
Based on our patient’s clinical findings (the presence of an optic pit in conjunction with a neurosensory serous retinal detachment and the absence of any underlying neoplastic, inflammatory or vascular etiologies), the patient was diagnosed with ODPM.
Treatment Options
One of the initial treatments for ODPM was the use of oral corticosteroids and acetazolamide. This treatment proved ineffective in a majority of cases; though the retinal fluid would be initially absorbed, the serous fluid would later reappear after the discontinuation of treatment. As a result, the use of oral corticosteroids and acetazolamide is no longer a valid treatment options.11
Another early treatment modality was the use of argon laser photocoagulation. Researchers suggest that laser scars, created temporally to the disc margin, would create a chorioretinal barrier that would prevent the passage of fluid from the optic pit to the underlying subretinal space.11
Though fluid reabsorption and retinal reattachment was reported in some patients, this treatment had a low initial success rate with visual field defects arising as a consequence.11,12 This was especially true in cases were serous maculopathy was present in conjunction with a retinoschisis.11,12 Though laser photocoagulation provides a strong adhesion between the photoreceptors and the RPE, this procedure alone fails to prevent intraretinal fluid migration.11
Intravitreal gas injection has also been proposed as a treatment option, although this is more successful when combined with laser photocoagulation.13,14
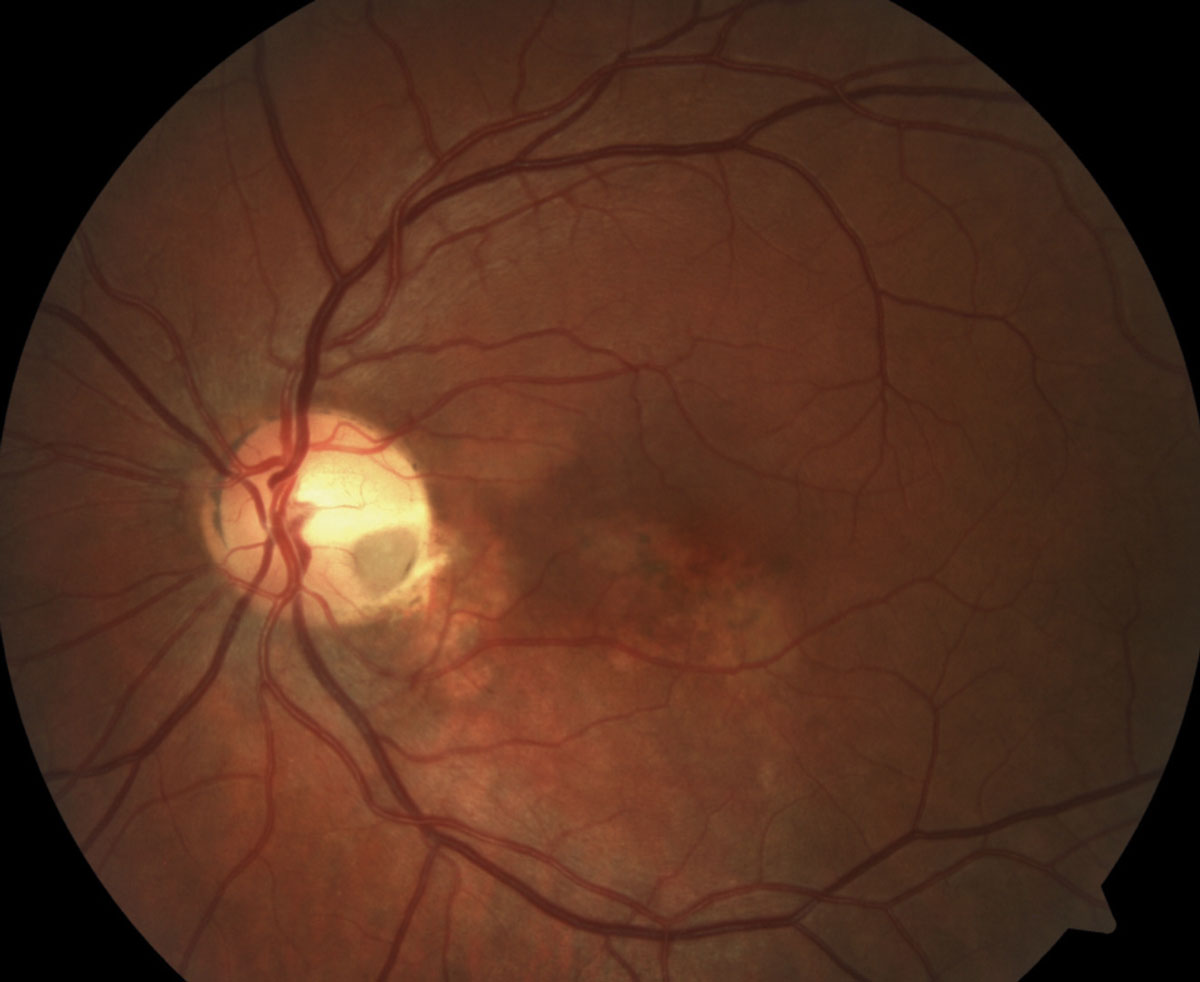 |
| This fundus image of another patient helps reveal that subretinal and intraretinal fluids are at the root of the reported central vision loss. Photo: Mohammad Rafieetary, OD. Click image to enlarge. |
Another option for these patients, macular buckling surgery, involves positioning a scleral sponge at the posterior aspect of the globe behind the macula along the 6 o’clock to 12 o’clock meridian. An intraoperative B-scan is required for exact placement of the macular buckle. Though this procedure is highly successful, its complexity has prevented it from gaining much popularity.15
The current literature favors pars plana vitrectomy (PPV) as the treatment of choice for ODPM. PPV is most commonly performed in combination with different surgical procedures such as barricade photocoagulation, intravitreal gas injection and internal limiting membrane (ILM) peeling.16 One study found the use of combination PPV, laser photocoagulation and intravitreal gas injection improvemed VA in 90% of patients with a complete resolution of maculopathy in 70%.17
PPV is generally performed within an hour or two of laser photocoagulation. Removing the vitreous gel during this time frame allows the gas bubble to compress the retinal layers juxtapapillary to the optic disc. This allows for formation of intraretinal scars during the seven-to-10 day postoperative face-down period, seizing the migration of intraretinal fluid.11,1
Researchers hypothesize that vitreous traction could be responsible for ODPM development. Much has been published on the resolution of ODPM following posterior vitreous detachment (PVD) induction with combined surgery. In one series, vitreous adhesion to the macular surface was noted during surgical intervention.18 Such literature has acted as supportive evidence for vitreous traction in the role of maculopathy development.
A 2004 study evaluated 11 patients who underwent PPV, PVD induction, laser photocoagulation and gas tamponade for ODPM and found a complete resolution of serous maculopathy in all cases with an 18% recurrence rate.19 In another, 11 patients underwent PPV, PVD induction and gas tamponade without laser.20 Complete resolution of maculopathy was noted in 91% of the patients with no reccurrences.20 Based on the previously mentioned series, the use of laser photocoagulation may not be required for surgical success.
One surgical method that has been surrounded by controversy is the use of ILM peeling. Although many researchers have advocated its use, the benefits of this surgical technique remain questionable. In one study, 10 patients underwent PPV, PVD induction, ILM peel and gas tamponade with laser photocoagulation. Complete resolution occurred in 50% of patients and 70% gained greater than or equal to two lines of VA. However, complications of macular holes were seen.21 In a different study, seven patients were treated in a similar manner and complete resolution was achieved in 86% of eyes with 71% reaching a final VA of greater than or equal to 20/30. In that case, four eyes resulted in full-thickness macular holes after one month.22
Possible indications for ILM peeling would be when maculopathy is found in conjunction with an epiretinal membrane or in the presence of overlying vitreoschsis. Given the number of cases that have been successfully performed in the absence of ILM peeling along with the relatively high incidence of macular hole development ILM peeling is generally avoided unless there is an underlying pathogenic role contributing to serous maculopathy due the ILM.11,20-24
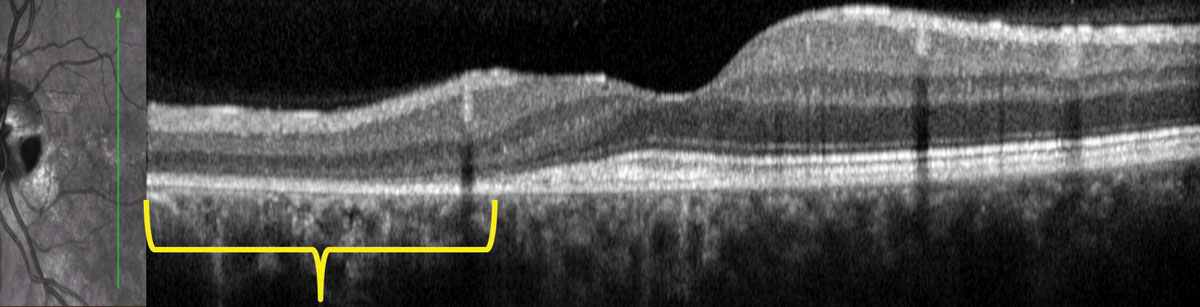 |
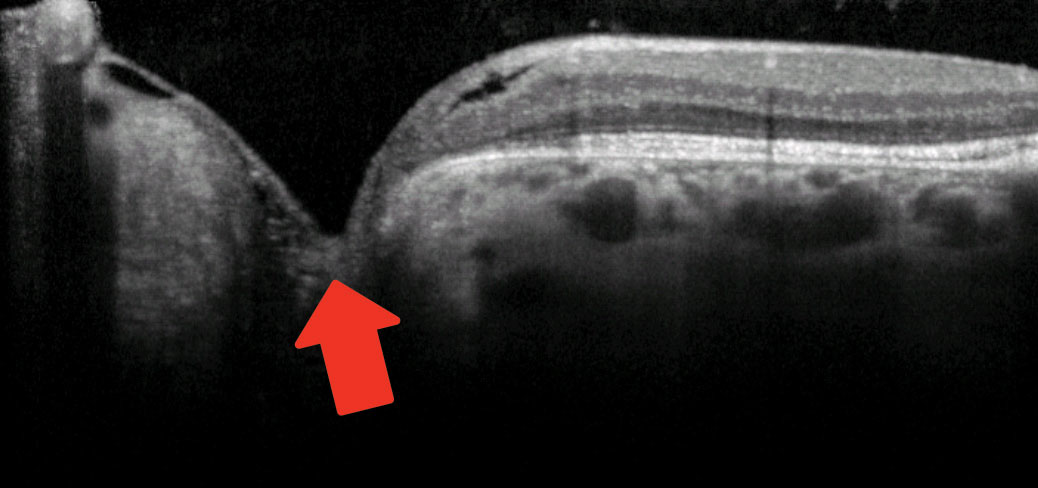 |
| OCT imaging of the patient seen above shows outer retinal disruption (in yellow brackets, top), indicative of possible serous detachment in the past. Below, cavitation in the area of the pit is shown with the red arrow. Photos: Mohammad Rafieetary, OD. Click images to enlarge. |
Some authors suggest the removal of any glial tissue overlying (or adjacent to) the optic pit. One study looked at nine patients undergoing PPV, PVD induction, laser photocoagulation and gas tamponade. Serous maculopathy resolved in six out of six patients in which the glial tissue surrounding the optic pit was removed. It only resolved in two out three patients in which the glial tissue was not removed.25 A 2008 case report demonstrated the benefits of glial tissue removal in a 45-year-old male who was diagnosed with ODPM. PPV, PVD induction and gas tamponade with glial tissue peeling was performed and retinal reattachment was achieved in six months with a final visual acuity of 20/20 without any visual field defects.26 Though glial tissue peeling has proven beneficial in a limited group of studies, precaution must be taken when performing this procedure due to the glial tissue’s close proximity to the optic nerve.25,26
Research suggests that sealing the CODP during surgery to prevent the passage of fluid into the intraretinal and subretinal spaces can prove beneficial.27 Other techniques used to seal a CODP include using an autolohous scleral flap, inverting peeled ILM and using a Tisseel fibrin sealant.6
Recently, investigators have proposed a new strategy in ODPM treatment—the creation of inner retinal fenestrations temporal to the optic disc. In theory, this procedure works by disturbing the pressure gradient, which pushes fluid from the inner retinal layers into the subretinal space. This creates retinal fenestrations that allow for the diversion of fluid back into the vitreous.
In one of the largest published series on ODPM treatment, investigators noted complete resolution of maculopathy in 94% of eyes with a final VA greater than or equal to 20/30 in 56% of eyes. Retinal fenestrations can close prematurely during the early postoperative period.28
Clinical Pearls
No established guidelines for the treatment of ODPM exist, nor has a clear consensus on the mechanism of pathogenesis or the origin of the subretinal fluid been reached.6,11 This makes it difficult to determine the optimal surgical technique. Typically, ODPM is treated with a combination of juxtapapillary retinal laser photocoagulation, PPV, gas tamponade and ILM peeling.16
Additional surgical elements may include subretinal drainage, glial tissue peeling, and sealing the CODP.11 PVD induction is worthwhile to perform to relieve any vitreoretinal traction.
Treatment with laser photocoagulation is never performed alone and macular buckling is rarely performed due to its level of complexity.15,18 Though PPV with intraretinal fenestrations have provided promising results but further studies are required.
Spontaneous resolution can occur in 25% of cases but most have a poor visual prognosis if left untreated.6,29 Patients presenting with ODPM should be referred to a retina specialist promptly. Prophylactic parapapillary laser has not been studied as a preventative measure against ODPM.30 Patients should be advised to return to clinic immediately if any visual changes are noted. Yearly comprehensive eye exams with the use of at-home Amsler gird monitoring should be considered.
Our patient was issued a new polycarbonate spectacle prescription for full-time wear. She was educated on the condition and referred to a retinal specialist for a consultation.
Due to the longstanding nature of the condition, a guarded visual prognosis was emphasized. The patient was then instructed to return to clinic in three months for another evaluation.
Dr. Corzo is a graduate of Illinois College of Optometry and an optometry resident at Nova Southeastern University College of Optometry where he specializes in primary care with an emphasis on ocular disease.
1. Kranenburg EW. Craterlike holes in the optic disc and central serous retinopathy. Arch Ophthalmol. 1960;64(12):912-24. 2. Jonas J, Freisler K. Bilateral congenital optic nerve head pits in monozygotic siblings. Am J Ophthalmol. 1997;124(6):844-6. 3. Johnson K. Optic disc pit: primary diagnosis in a child. Clin Exp Optom. 2006;89(4):257-9. 4. Stefko S, Campochiaro P, Wang P, et al. Dominant inheritance of optic pits. Am J Ophthalmol. 1997;124(1):112–3. 5. Brown G, Shields J, Goldberg R. Congenital pits of the optic nerve head: II. Clinical studies in humans. Ophthalmology. 1980;87(1):51-65. 6. Moisseiev E, Moisseiev J, Loewenstein A. Optic disc pit maculopathy: when and how to treat? A review of the pathogenesis and treatment options. Int J Retina Vitreous. 2015;1(1):3. 7. Chatziralli, I, Theodossiadis P, Theodossiadis G. Optic disc pit maculopathy: current management strategies. Clin Ophthalmol. 2018;12:1417-22. 8. Brodsky M. Congenital optic disk anomalies. Surv Ophthalmol. 1994;39:89-112. 9. Sobol W, Blodi C, Folk J, Weingeist T. Long-term visual outcome in patients with optic nerve pit and serous retinal detachment of the macula. Ophthalmol. 1990;97:1539–42. 10. Ehlers J, Shah CP (eds). The Wills Eye Manual Office and Emergency Room Diagnosis and Treatment of Eye Disease. 5th ed. Boston: Lippincott Williams & Wilkins; 2008:282-3. 11. Nieraj J, Johnson M. Pathogenesis and treatment of maculopathy associated with cavitary optic disc anomalies. Am J Ophthalmol. 2014;158(3):423-35. 12. Brockhurst R. Optic pits and posterior retinal detachment. Trans Am Ophthalmol Soc. 1975;73:264-91. 13. Akiyama H, Shimoda Y, Fukuchi M, et al. Intravitreal gas injection without vitrectomy for macular detachment associated with an optic disc pit. Retina 2014;34(2):222-7. 14. Lei L, Li T, Ding X, et al. Gas tamponade combined with laser photocoagulation therapy for congenital optic disc pit maculopathy. Eye (Lond). 2015;29(1):106-14. 15. Theodossiadis G, Theodossiadis P. The macular buckling technique in the treatment of optic disk pit maculopathy. Semin Ophthalmol. 2000;15(2):108-15. 16. Georgalas I, Ladas I, Georgopoulos G, Petrou P. Optic disc pit: a review. Graefes. Arch Clin Exp Ophthalmol. 2011;249(8):1113-22. 17. Taiel-Sartral M, Mimoun G, Glacet-Bernard A, et al. Vitrectomy-laser-gas for treating optic disc pits complicated by serous macular detachment. J Fr Ophtalmol. 1996;19(10):603-9. 18. Bartz-Schmidt K, Heimann K, Esser P. Vitrectomy for macular detachment associated with optic nerve pits. Int Ophthalmol. 1995-1996;19(6):323–9. 19. García-Arumí J, Guraya B, Espax A. Optical coherence tomography in optic pit maculopathy managed with vitrectomy-laser-gas. Graefes Arch Clin Exp Ophthalmol. 2004;242(10):819–26. 20. Hirakata A, Okada AA, Hida T. Long-term results of vitrectomy without laser treatment for macular detachment associated with an optic disc pit. Ophthalmol. 2005;112(8):1430-5. 21. Rizzo S, Belting C, Genovesi-Ebert F, et al. Optic disc pit maculopathy: the value of small-gauge vitrectomy, peeling, laser treatment, and gas tamponade. Eur J Ophthalmol. 2012;22(4):620-5. 22. Shukla D, Kalliath J, Tandon M, Vijayakumar B. Vitrectomy for optic disc pit with macular schisis and outer retinal dehiscence. Retina. 2012;32(7):1337-42. 23. Hirakata A, Inoue M, Hiraoka T, McCuen B. Vitrectomy without laser treatment or gas tamponade for macular detachment associated with an optic disc pit. Ophthalmol. 2012;119(4):810-8. 24. Avci R, Yilmaz S, Inan U, et al. Long-term outcomes of pars plana vitrectomy without internal limiting membrane peeling for optic disc pit maculopathy. Eye (Lond). 2013;27(12):1359-67. 25. Gregory-Roberts E, Mateo C, Corcóstegui B, et al. Optic disc pit morphology and retinal detachment: optical coherence tomography with intraoperative correlation. Retina. 2013;33(2):363–70. 26. Inoue M, Shinoda K, Ishida S. Vitrectomy combined with glial tissue removal at the optic pit in a patient with optic disc pit maculopathy: a case report. J Med Case Reports. 2008;2(1):103. 27. Rosenthal G, Bartz-Schmidt K, Walter P, Heimann K. Autologous platelet treatment for optic disc pit associated with persistent macular detachment. Graefes Arch Clin Exp Ophthalmol. 1998;236:151–3. 28. Ooto S, Mittra R, Ridley M, Spaide R. Vitrectomy with inner retinal fenestration for optic disc pit maculopathy. Ophthalmol. 2014;121(9):1727-33. 29. Yuen CH, Kaye SB. Spontaneous resolution of serous maculopathy associated with optic disc pit in a child: a case report. J AAPOS. 2002;6:330-1. 30. Jain N, Johnson M. Pathogenesis and treatment of maculopathy associated with cavitary optic disc anomalies. Am J Ophthalmol. 2014;158(3):423-35. |

