AGE RELATED MACULAR degeneration (AMD) is the leading cause of vision loss among older Americans, with over ten million suffering from the disorder and nearly two million suffering with wet AMD.1 Over the past several years, we have seen major breakthroughs in the treatment of wet AMD. Because up to 90% of cases of severe vision loss are caused by choroidal neovascularization (CNV), this is indeed great news.2,3
Understanding the causes of dry AMD and learning how to treat it at an earlier stage are the next big challenges facing clinicians and researchers. The clinical director of the National Eye Institute (NEI), Frederick L. Ferris III, recently emphasized this fact by stating that, “The blood vessels of wet AMD are like weeds in a garden. You have a choice: You can either be picking the weeds or making a healthy garden so weeds can’t grow.”
We are gradually starting to understand what causes an “unhealthy garden.” It is thought that oxidative stress first causes injury of the retinal pigment epithelial (RPE) and possibly the choriocapillaris. This injury results in a chronic inflammatory response within Bruch’s membrane and the choroid, which is followed by the formation of an abnormal extracellular matrix (ECM). This, in turn, causes altered diffusion of nutrients to the retina and RPE, which may cause further damage. The abnormal ECM itself may also lead to geographic atrophy (GA) and/or to CNV.
These processes may not happen in a sequential fashion. It is also expected that an individual’s environment and genetic makeup alter susceptibility to AMD.4
Several clinical trials for dry AMD are testing whether the processes described above can be slowed down, stopped or reversed. These trials include the Age Related Eye Disease 2 Study (AREDS 2) and trials testing an intra-ocular implant that delivers ciliary neurotrophic factor (CNTF); a capsule containing fenretinide; an eyedrop containing OT-551; an intravitreal injection of POT-4; a subcutaneous injection of glatiramer acetate; a juxtascleral injection of anecortave acetate; selective RPE laser treatment, RPE transplantation and Rheopheresis. New genetics data has also recently shown an important role of toll-like receptor TLR3 in the susceptibility to dry AMD with important implications for both current and future treatments of dry and wet AMD.
AREDS 2
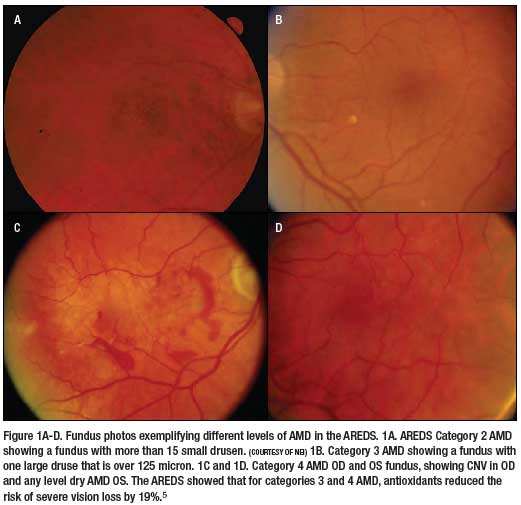
To put AREDS 2 in the correct perspective, you must go back to the original AREDS. AREDS tested the concept that inhibition of oxidative damage to the RPE can reduce the risk of AMD. In this study, patients received 15mg betacarotene, 500mg vitamin C, 400 international units of vitamin E, 80mg of zinc and 2mg of cupric oxide (Figure 1).5 And indeed, results from this landmark study published in 2001 showed that for patients with moderate dry AMD and patients with severe AMD in one eye, the chance of severe vision loss could be reduced by 19%.
Finally, eye care practitioners had something to offer to patients with dry AMD. As a result, offering antioxidant supplements to AMD patients has now become commonplace in the eye care practice.
However, several issues remained. One was that these supplements only had a moderate impact (19% reduction) on prevention of severe vision loss. Second, the supplements were not shown to prevent development of milder forms of AMD. Third, the intake of high levels of beta-carotene has been shown to increase the risk of lung cancer in smokers.6 Supplements that replace beta-carotene with lutein (e.g. Preservision Lutein, Bausch & Lomb) have subsequently been made available to patients. And fourth, AREDS itself revealed that other dietary components might play an even larger role in preventing AMD than anti-oxidants.
Specifically, AREDS showed that increased dietary intake of the macular xanthophylls lutein and zeaxanthin, omega-3 long-chain polyunsaturated fatty acid (LCPUFA) and fish decreased the likelihood of having CNV. AREDS also revealed that dietary intake of macular xanthophylls decreased GA and large or extensive intermediate drusen, while dietary intake of the antioxidants vitamins A, C, and E did not seem to impact the overall risk of AMD.7,8
Two other studies similarly revealed that both dietary intake and supplementation of antioxidants did not significantly impact the prevention of early AMD, while the intake of omega-3 LCPUFA and fish did have a significant impact.9,10
Finally the Lutein Antioxidants Supplement Trial (LAST) showed vision improvement in AMD patients.11 Both prevention of early AMD and vision improvement had not been shown in the AREDS. While lutein and zeaxanthin may protect the macula from oxidative damage, omega-3 LCPUFAs have been shown to yield anti-inflammatory activity, indicating that inflammation may play just as much of a role in the pathogenesis of AMD.12
Inflammatory cells have been shown to be present in the retinas of patients with AMD, and genetic markers associated with AMD are part of the complement system.13 Blood levels of C-reactive protein have also been shown to be high in patients with AMD.14 AREDS also looked at non steroidal anti-inflammatory drug (NSAID) use and determined that it reduced the risk of developing central geographic atrophy.15
AREDS 2 may answer whether supplementation with omega-3 LCPUFA and macular xanthophylls is a more effective and safer means of preventing both dry and wet AMD. AREDS 2 is a multi-center, randomized trial designed to assess the effects of oral supplementation of the macular xanthophylls lutein and zeaxanthin and/or the omega-3 LCPUFAs docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) on the progression to advanced AMD. An additional goal of the study is to test AREDS nutritional supplements with reduced zinc or no beta-carotene. AREDS 2 is a 5-year, double-blind study that has enrolled nearly 40,000 patients age 50 to 85 at nearly 100 centers nationwide since 2006.
Ciliary Neurotrophic Factor
Ciliary neurotrophic factor (CNTF) is a human gene that is part of the Interleukin-6 family of cytokines.16 The protein encoded by this gene is a polypeptide hormone and nerve growth factor that promotes neurotransmitter synthesis and neurite outgrowth in the nervous system. CNTF enhances survival of neurons and oligodendrocytes, and may reduce inflammatory tissue destruction. For this reason, it has been postulated to be an important factor in neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS) and AMD. CNTF was found to be effective in retarding vision loss from photoreceptor cell death in 13 animal models of outer retinal degeneration.
 The first possible medical application
of CNTF was discovered
in a human study in 2001.
This study, testing the
usefulness of CNTF for
the treatment of ALS,
revealed that CNTF produced
significant weight
loss in the study subjects
without significantly affecting
the course of ALS.17 It
was later established that
CNTF could reduce food
intake without causing
hunger or stress, acting through a nonleptin
pathway.18
The first possible medical application
of CNTF was discovered
in a human study in 2001.
This study, testing the
usefulness of CNTF for
the treatment of ALS,
revealed that CNTF produced
significant weight
loss in the study subjects
without significantly affecting
the course of ALS.17 It
was later established that
CNTF could reduce food
intake without causing
hunger or stress, acting through a nonleptin
pathway.18
In 2003, Regeneron tested a modified and more potent version of CNTF––Axokine––as a drug for obesity. In this trial, two-thirds of patients developed antibodies against Axokine. Since this could potentially interfere with the neuroprotective effect of endogenous CNTF, the drug was not commercialized.
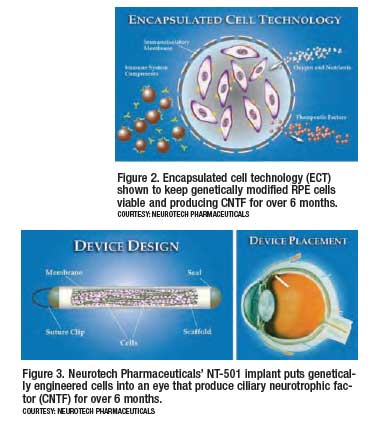
However, since the vitreous is immunologically inert, CNTF can be applied locally to the macula without the concern of systemic side-effects. NEI-sponsored phase I and phase II clinical trials have been completed, which test the safety and efficacy of intraocular implants that deliver CNTF to the macula in patients with AMD.19-21 In these trials, human RPE cells that have been given the ability to make CNTF were placed in an encapsulated cell technology ([ECT], see figures 2 and 3) intraocular implant called NT-501 (Neurotech Pharmaceuticals) and tested in patients for six months. At the end of the study period, the implants contained viable cells that still produced CNTF at what is considered therapeutic levels.
While this study was done in patients with retinitis pigmentosa, it is encouraging that three out of seven tested eyes had a two-to three-line improvement in visual acuity. Since then, two phase II clinical trials have been initiated that test the NT-501 implant in patients with atrophic AMD. One trial sponsored by Neurotech Pharmaceuticals was started in January 2007 at eight clinic sites across the United States. Another trial sponsored by the NEI at the National Institute of Health in Bethesda, Md., started enrollment in January of 2006. Both studies have followed patients over an 18-month period, remove the device after 12 months, include a high dose, low dose and sham group, have as primary outcome measure best corrected visual acuity, and include a combined total of 84 patients. Results should be forthcoming in the near future.
Fenretinide
Fenretinide (Sirion Therapeutics) is a retinoic acid derivative that is thought to reduce the accumulation of lipofuscin in the retina. Lipofuscine is a major component of the abnormal extracellular matrix, which mainly accumulates in the form of drusen associated with AMD. Fenretinide binds to retinol (vitamin A), which keeps it from entering the outer retina. Retinol is a precursor to rhodopsin. The breakdown of rhodopsin may produce toxic byproducts, such as lipofuscin. Thus, less available retinol may lead to less toxic byproducts and reduce the risk of dry AMD. Fenretinide is taken as a once-a-day capsule. Sirion Therapeutics has sponsored a Phase II clinical trial that enrolled 225 patients over a two-year period. Three equal groups of patients receive either 100 mg of fenretinide, 300 mg of fenretinide, or a placebo.22 Preliminary results are expected in late 2008.
OT-551
Othera Pharmaceuticals, Inc. has developed a drug called OT-551 that the company describes as a small molecule with primarily anti-oxidant, but also anti-inflammatory and anti-angiogenic properties. The drug is applied as an eye drop that penetrates to the retina and choroid. Two trials that test OT-551 for chronic treatment of dry AMD are ongoing. The first is a NEI-sponsored pilot study of 10 patients that was initiated in 2006 and tests the effect of 0.45% OT-551 drops taken three times a day over a period of three years. Primary outcomes measured are VA, with area of GA as a secondary measure.23
A second, 18-site Phase II study sponsored by Othera Pharmaceuticals which plans to enroll 198 participants with bilateral geographic atrophy was started in 2007. This study is expected to be completed in 2010, and intends to characterize the effect of 0.45% and 0.3% concentration of OT-551 eye drops given three times a day on the progression of geographic atrophy area over a two-year period.24
POT-4
Potentia Pharmaceuticals’ first product, POT-4, is a complement component C3 inhibitor that is formulated to be dosed less frequently than currently approved intravitreal injections for AMD. POT-4 falls in the category of treatments that act on inflammation associated with AMD. In 2007, Potentia initiated a one year, Phase I trial of POT-4 at six clinical sites in 15 patients with subfoveal CNV. The company also intends to conduct clinical trials in patients with geographic atrophy.25
Glatiramer Acetate
Glatiramer Acetate (Copaxone®, Teva Pharmaceutical Industries) is an immunomodulatory substance that has been shown to reduce cognitive decline, eliminate plaque formation, and induce neuron survival and neurogenesis in a mouse model for Alzheimer’s disease (AD). It has been tested extensively in humans with multiple sclerosis (MS), ALS and Crohn’s disease, and is approved for use in patients with relapsing- remitting MS.
AD and AMD are both strongly correlated with age and demonstrate accumulation of extracellular matrix––amyloid plaques in AD and drusen in AMD. Additionally, inflammatory mediators and activated microglia are present in amyloid deposits, as well as in drusen.
Copaxone® is applied as a subcutaneous injection. Two double blind, randomized clinical trials at the New York Eye & Ear Infirmary and the Kaplan Medical Center, Rehovot, Israel, have been initiated in 2006 and 2007 respectively, and are enrolling up to 60 patients combined. The primary outcome tested in these trials is the reduction in the total area of drusen.26,27 Results have not been published yet.
Selective RPE Laser Treatment
For the past 20 years, reports on treatment of drusen with laser have provided mixed results. While studies such as the Choroidal Neovascularization Prevention Trial (CNVPT), the Complications of Age-Related Macular Degeneration Prevention Trial (CAPT) and the Drusen Laser Study clearly showed that drusen resolve after laser treatment, the overall visual benefit and the absence of side effects still have to be determined in a convincing manner.28
A clinical trial at the University of Regensburg, Germany from 2004 to 2006, aimed to enroll 60 patients with drusen, GA and other macular diseases for treatment with short pulses of laser that specifically destroy the RPE, while not damaging surrounding tissues, such as photoreceptors. In theory, this treatment removes sick RPE and replaces it with new RPE, creating a healthier RPE layer and improving retinal function. No results are yet available for this study.
RPE Transplantation
Instead of interfering with the disease process, another potential treatment replaces damaged and unhealthy RPE with healthy tissue. In a 10-patient (four had AMD) study published this past July, human neural retinal progenitor cell layers and RPE were transplanted into eyes with vision of 20/200 or worse. All four patients with AMD experienced improved visual acuity, even though none improved to better than 20/200. There was no graft rejection during a follow-up time of up to six years despite the lack of a perfect match in all cases.29
Anecortave Acetate
Anecortave acetate (Retaane, Alcon) is applied as a posterior juxtascleral depot injection and was initially developed to treat active CNV. The Anecortave Acetate Risk-Reduction Trial (AART) was initiated to test whether the risk for CNV can be reduced in patients with dry AMD.30 This Alcon-sponsored phase III clinical trial tested 15mg, 30mg or sham injections of the angiostatic steroid every six months for 48 months. In the study, 2,546 patients had dry AMD in one eye and CNV in the other eye.
In July of 2008, Alcon revealed that it would not continue development of Anecortave acetate for dry AMD because clinical trial results showed no reduction in the progression from dry to wet AMD and no change in the time it took for the development of sight threatening CNV.
Rheopheresis®
Developed by OccuLogix, Rheopheresis ® is the name for double filtration plasmapheresis that uses plasma filtration filters specifically designed for patients with dry AMD. The filtration process removes large proteins, fats and other substances from the blood. This, in theory, improves blood flow to the macula and enhances diffusion of nutrients to the retina and RPE. In 2002, interim data published on the first 43 patients (28 test, 15 placebo) enrolled in the Multicenter Investigation of Rheopheresis® for AMD Study (MIRA- 1) revealed a significant beneficial effect in treated patients with mild and moderate dry AMD. MIRA-1 patients received eight treatments over 10 weeks.
The results showed that 13% of treated versus 0% percent of placebo-control eyes had three lines or more of improvement in best-corrected visual acuity (BCVA) at 12 months. Also, only 4% of treated versus 18% of placebo-control eyes had three lines or more loss of BCVA. This result was significant and even more pronounced in the subgroup of patients with baseline BCVA worse than 20/40.31 More definitive conclusions may be made once results from the full 180 patients that the study aims to enroll are available.32 As of now, this data has not been published.
Occulogix has also sponsored the Safety and Effectiveness Investigation for Dry, Non-Exudative Age Related Macular Degeneration Using Rheopheresis Study (RHEO-AMD), which is of a design similar to MIRA-1 y and aims to enroll 325 patients at 39 clinic sites. However, this study was reported to have been halted “due to the financial position” of Occulogix.33 However, the Occulogix Web site shows that the company is still operating.
Toll-like Receptor 3
Toll-like receptor 3 (TLR3) plays a role in innate immunity and host defense, and alerts the immune system to viral infection. TLR3 may cause destruction of RPE cells when activated. In a large study, patients with less active variants of TLR3 had a reduced risk of developing GA, but no difference in the risk of CNV. The same study also showed that mouse and human RPE cells were more likely to die in tissue culture when TLR3 is activated. These results have two important implications for AMD treatments. First, TLR3 inhibitors may be used in the future to stop the formation of GA. Second, these results serve as a caution for current experimental treatments of CNV with short-interfering- RNA (siRNA), because TLR3 is activated by the presence of viral RNA, it may also be activated by siRNA causing GA while trying to protect against CNV.34
Summary
It was not that long ago that patients with subfoveal CNV were treated with thermal laser that resulted in immediate loss of vision and questionable functional benefit. We now have effective treatments for CNV that give hope to many patients. However, vision loss from GA still cannot be treated and dry AMD cannot be halted at an early stage or before it develops.
Current clinical trials for dry AMD test treatments that affect all the different stages proposed. AREDS 2 and OT- 551 target the oxidative stress that leads to injury of the RPE and possibly the choriocapillaris. AREDS 2, CNTF, OT- 551, POT-4, glatiramer acetate and TLR3 inhibitors home in on the subsequent chronic inflammatory response within Bruch’s membrane and the choroid. Fenretinide and glatiramer acetate affect the formation of an abnormal extracellular matrix that follows, while Rheopheresis attempts to then reverse the altered diffusion of nutrients to the retina and RPE. The final stage of further damage to the RPE (GA) is attempted to be remedied in the selective RPE laser treatment and RPE transplantation trials.
At least some of these trials are expected to produce applicable results in the coming two to three years. It is important for the primary eye care provider to follow developments closely as these outcomes will significantly affect what is done in the optometric office.
Dr. Stokkermans is a full time assistant professor at the Case Western Reserve University School of Medicine, Department of Ophthalmology and Visual Sciences, and sees patients at University Hospitals Case Medical Center, Cleveland, Ohio. He has published and lectured on AMD in the past.
References
- Congdon N, O’Colmain B, Klaver CC, et al, Mitchell P; Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004 Apr;122(4):477-85.
- Hyman LG, Lilienfeld AM, Ferris FL III, et al. Senile macular degeneration: A casecontrol study. Am J Epidemiol 1983 118:213-27.
- Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 1997;104:7-21.
- Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol 2004;122:598-614.
- A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for agerelated macular degeneration and vision loss. AREDS Report No. 8. Arch Ophthalmol 2001;119:1417-36.
- Smigel K. Beta carotene fails to prevent cancer in 2 major studies: CARET intervention stopped. J Natl Cancer Inst 1996 Feb 21;88(3-4):145.
- The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS report no. 20. Arch Ophthalmol 2007;125:671-9.
- SanGiovanni JP, Chew EY, Clemons TE, et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol 2007 Sep;125(9):1225-32.
- Chong EW, Kreis AJ, Wong TY, et al. Dietary omega-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: a systematic review and meta-analysis. Arch Ophthalmol 2008 Jun;126(6):826-33.
- Chong EW, Wong TY, Kreis AJ, et al. Dietary antioxidants and primary prevention of age related macular degeneration: systematic review and meta-analysis. BMJ 2007 Oct 13;335(7623):755. Epub 2007 Oct 8.
- Richer S, Stiles W, Statkute L, et al. Double-masked, placebocontrolled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004 Apr;75(4):216-30.
- Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr 2002 Dec;21(6):495-505.
- Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. PNAS 2005; 102:7227-32.
- Seddon JM, Gensler G, Milton RC, et al. Association between Creactive protein and age-related macular degeneration. JAMA 2004; 291:704-10.
- Age-Related Eye Disease Study Research Group. Risk factors for the incidence of advanced age-related macular degeneration in the Age-Related Eye Disease Study (AREDS): AREDS Report No. 19. Ophthalmology 2005; 112:533-9.
- National Center for Biotechnology Information, Entrez Gene database: Ciliary neurotrophic factor, CNTF.
- Bongioanni P, Reali C, Sogos V. Ciliary neurotrophic factor (CNTF) for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev 2004;(3):CD004302.
- Lambert PD, Anderson KD, Sleeman MW, et al. Ciliary neurotrophic factor activates leptin-like pathways and reduces body fat, without cachexia or rebound weight gain, even in leptin-resistant obesity. Proc Natl Acad Sci U.S.A. 2001;98(8):4652–7.
- Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006 Mar 7;103(10):3896-901.
- A Phase II Study of Implants of Encapsulated Human NTC-201 Cells Releasing Ciliary Neurotrophic Factor (CNTF) in Participants With Visual Acuity Impairment Associated With Atrophic Macular Degeneration. Clinicaltrials.gov, identifier NCT00277134.
- A Study of an Encapsulated Cell Technology (ECT) Implant for Patients with Atrophic Macular Degeneration. Clinicaltrials.gov, identifier NCT00447954.
- Study of Fenretinide in the Treatment of Geographic Atrophy Associated With Dry Age-Related Macular Degeneration ClinicalTrials.gov, identifier NCT00429936
- OT-551 Antioxidant Eye Drops to Treat Geographic Atrophy in Age-Related Macular Degeneration. ClinicalTrials.gov identifier NCT00306488.
- The OMEGA Study: Use of Eye Drops to Treat Geographic Atrophy Associated With Age-Related Macular Degeneration (Dry AMD) ClinicalTrials.gov identifier: NCT00485394.
- Safety of Intravitreal POT-4 Therapy for Patients With Neovascular Age-Related Macular Degeneration. Clinicaltrials.gov, identifier NCT00473928.
- Weekly Vaccination With Copaxone as a Potential Therapy for Dry Age-Related Macular Degeneration. Clinicaltrials.gov, identifier NCT00541333.
- Copaxone in Age Related Macular Degeneration. Clinicaltrials. gov, identifier NCT00466076.
- Stokkermans TJW. Treatment of age-related macular degeneration, Clin Eye Vis Care 2000;12(1):15-35.
- Radtke ND, Aramant RB, Petry HM, et al. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. AJO 2008;146(2):172-82.
- Anecortave Acetate Risk-Reduction Trial (AART). Clinicaltrials.gov, identifier NCT00307398.
- Pulido JS, Multicenter Investigation of Rheopheresis for AMD (MIRA-1) Study Group. Multicenter prospective, randomized, doublemasked, placebo-controlled study of Rheopheresis to treat nonexudative age-related macular degeneration: interim analysis. Trans Am Ophthalmol Soc 2002;100:85-106; discussion 106-7.
- Rheopheresis Blood Filtration Study for the Treatment of Dry Age-Related Macular Degeneration (AMD). Clinicaltrials.gov, identifier NCT00078221
- Safety and Effectiveness Investigation for Dry, Non-Exudative Age Related Macular Degeneration (AMD) Using Rheopheresis (RHEO-AMD) Clinicaltrials.gov, identifier NCT00460967.
- Z Yang. Toll-like receptor 3 and geographic atrophy in age-related macular degeneration N Engl J Med 2008. (E-pub ahead of print.)

