Six years into the first major study of keratoconus, weve learned many things about this disease. Data indicate that keratoconus is a bilateral but asymmetrical disorder, and that the vast majority of patients wear rigid lenses. Keratoconus is an inherited disease with variable penetrance. The average self-reported age of diagnosis is 279 years.
These are just some of the preliminary findings from the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Observational Study. This observational study, conducted at 15 clinical sites in the United States, is the first multi-center study designed and implemented by optometry that the National Eye Institute (NEI), a division of the National Institutes of Health, has funded. Originally funded for three years of annual follow-up, the NEI extended the study to eight years of follow-up.
Here is a review of some of the published literature on the CLEK Study, and a discussion about what those findings could mean in clinical practice.
The CLEK Study Explained
What Weve Learned
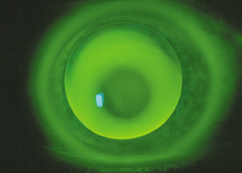
An apical touch fluorescein pattern.
These facts about the CLEK Study and its findings have been documented in the literature:
Baseline findings. Some 56% of the study subjects were male, and 13% of all subjects were aware of at least one close relative (father, mother, brother, sister, child, uncle or aunt) with keratoconus. Approximately one-half reported rubbing one or both eyes vigorously, and 53% said they had hay fever or allergies, 15% asthma and 8% atopic dermatitis.1 For comparison, atopic conditions have been reported in 10 to 20% of the general population.
At the baseline visit, 65% of subjects wore rigid contact lenses. Seventy-three percent of this group said their lenses were comfortable.1
Also, 86% of the subjects presented with a Fleischers ring in one (30%) or both (56%) eyes; Vogts striae in one (35%) or both (30%) eyes; and corneal scarring in one (31%) or both (22%) eyes.1
Repeatability of refraction. Each study subject received a manifest refraction and contact lens over-refraction (for patients wearing RGP lenses) at each annual CLEK Study visit. Following the baseline visit, the CLEK Study Coordinating Center randomly selected a prospective sample of 138 subjects to return for a repeat study visit, following the same testing protocol at each visit. Of those patients brought in for repeat visits, 92 were wearing RGP lenses with similar parameters at both visits.2
The median time between baseline and repeat visits was 90 days (the actual range was 22-268 days). The mean manifest refraction at baseline was: sphere -6.405.59D, cylinder -2.361.58D. The mean difference between baseline and repeat visit (repeat minus baseline values) manifest refractions was sphere -0.322.91D, cylinder -0.17
The purpose of the CLEK Study is to prospectively characterize the changes in vision, corneal curvature, corneal scarring and quality of life in patients with keratoconus, and to determine the factors associated with those changes over time. Patients do not receive treatment with contact lenses in the CLEK Observational Study. Instead, CLEK Study patients receive their contact lens fitting from their own practitioners outside of the study visits.
After the study coordinators and investigators trained co-investigators and staff and certified study protocols, the first of 1,209 study eligible keratoconus patients was enrolled in May 1995. Patient enrollment continued for 13 months. The study is now completing its sixth year of follow-up.
Eligibility criteria for the study required that the patient exhibit corneal distortion (distorted keratometry mire, scissors motion on retinoscopy or distortion of the red reflex on ophthalmoscopy) and at least one slit lamp sign of keratoconus. Positive slit lamp signs for study inclusion were the presence of a full or partial Fleischers ring, Vogts striae or corneal scarring indicative of keratoconus. Patients also had to have been at least 12 years old, had not received bilateral corneal transplants, and had been free of other ocular diseases that might result in reduced visual acuity.
Study patients were examined at a baseline visit and annually thereafter. The testing protocol, which takes approximately 2 1/2 hours to complete, consists of:
Patient-reported history.
Quality-of-life questionnaires (36-Item Short Form Health Survey and NEI Vision Functioning Questionnaire).
Entrance high- and low-contrast visual acuity through habitual correction.
Best-corrected high- and low-contrast visual acuity (patient wearing rigid contact lenses with optimal over-refraction Photodocumentation of the patients habitual rigid contact lens fitting relationship.
Contact lens verification.
Keratometry.
Manifest refraction and resultant high- and low-contrast visual acuity.
Photodocumentation of the flattest rigid contact lens from the CLEK Study trial lens set to show apical clearance (the first definite apical clearance lens (FDACL).
Slit lamp biomicroscopy.
Photodocumentation of the cornea.
Corneal topography.
1.39D. The mean contact lens over-refraction was sphere
0.330.89D, cylinder -0.55 0.68D. The mean difference between baseline and repeat visit over-refractions was sphere -0.11
0.81D, cylinder 0.020.67D. The mean absolute difference for axis was 18.120.2 degrees for the manifest refractions and 11.69.9 degrees for the over-refractions.2
RGP lens fitting. The CLEK investigators reported that 11.8% of the subjects in RGP lenses were wearing lenses fitted with apical clearance, and 88.2% were wearing lenses fitted with apical touch. For mild (steep keratometric reading <45.00D) keratoconic corneas, the average estimate of the base curve-to-cornea fitting relationship was 1.18D flat (SD1.84D); moderate (steep keratometric reading 45.00D to 52.00D) corneas were fitted on average 2.38D flat (SD2.56D); and severe (steep keratometric reading >52.00D) corneas were fitted an average of 4.01D flat (SD4.11D). Overall, RGP lenses were fitted on average 2.86D (SD 3.31D) flatter than the first definite apical clearance lens (FDACL)the flattest trial lens that shows an apical clearance fluorescein pattern.3
Apical clearance fitting. In a pilot study conducted prior to the CLEK Observational Study, investigators randomly assigned 17 of 30 keratoconic patients to a steep contact lens fitting regimen. All patients were successfully fitted initially with apical clearance (0.2mm steep) lenses. One of 22 eyes completing this pilot study developed mild corneal scarring (one patient was lost to follow-up at three months, and three were lost to follow-up at one year).4
Corneal scarring. Baseline study data suggest that corneal scarring in keratoconus is associated with five factors: corneal staining, contact lens wear, the presence of Fleischers ring, a steeper cornea and increasing age. Although many of these factors relate to the severity of the disease, clinical intervention in optimizing the contact lens fitting characteristics may affect the degree of corneal staining.5
What it All Means
The CLEK Observational Study will continue to collect data for two more years. So, data investigating the long-term effects of the disease have not been fully collected and analyzed.
Baseline data provide us with helpful insights to educate our keratoconus patients. Even though many researchers believe that keratoconus is an inherited disease, it exhibits poor penetrance in terms of clinically significant occurrence; 13% of the study patients have one or more family members who have been diagnosed with keratoconus. Because the average self-reported age of diagnosis was 279 years, many keratoconus patients are at a prime age for having children. Thus, it is important that they are counseled that it is unlikely that their child will become keratoconic.
Five percent of CLEK Study patients exhibited steep keratometric readings flatter than 45.00D. Although clinicians should suspect the possibility of keratoconus if keratometric readings are steeper than 48.00D, they should not assume that a patient with relatively flat corneas is not keratoconic. Corneal distortion and slit lamp signs are more indicative of the diagnosis of keratoconus than a steep cornea alone.
Manifest refraction is challenging for both the keratoconus patient and his or her doctor. The CLEK Study found that at the repeat visit (median 90 days after baseline visit), only 36% of patients had a sphere component of the manifest refraction that fell within 0.50D of the baseline refraction. It is still important to provide keratoconus patients with a current spectacle prescription in order to allow them to reduce their contact lens wearing time, and to function visually if they cannot wear their contact lenses due to an eye infection or irritation.
A Look into the Future
The CLEK Observational Study is scheduled for eight years of annual follow-up, concluding in 2004. The Coordinating Center at Washington University Medical School in St. Louis will compile and statistically analyze the longitudinal data. Then, the CLEK Study Group will prepare papers and presentations sharing the study results with practitioners.
The CLEK Study Group plans on submitting a grant proposal to the NEI for funding to conduct a clinical trial of rigid contact lens treatment options. A possible inter- vention might study the success of the apical touch fitting approach vs. the apical clearance method in keratoconus patients.
The follow-up study would use the results from the CLEK Observational Study to help determine sample size, intervention protocols and other study criteria. n
Dr. Edrington is a co-investigator of the CLEK Study team at the Southern California College of Optometry in Fullerton, Calif. He is also a member of the CLEK Study Executive Committee.
Acknowledgement: The CLEK Study is supported by the National Eye Institute/National Institutes of Health, grants EY10419, EY10069, EY10077, EY12656 and EY02687. It also was supported by Conforma Contact Lenses, Paragon Vision Sciences, CIBA Vision/Novartis Corporation and the Ohio Lions Eye Research Foundation.
CLEK Study Group Clinical Centers University of Alabama at Birmingham School of Optometry, Birmingham, Ala.: William Benjamin, O.D. Ph.D. (principal investigator); Carol Rosenstiel, O.D.; Maria Voce. Resource Centers Committees
University of California School of Optometry, Berkeley, Calif: Nina Friedman, O.D., M.S. (principal investigator); Dennis Burger, O.D.; Kelly McCann, M.F.A.
University Hospitals of Cleveland and Case Western Reserve University, Department of Ophthalmology, Cleveland: Loretta Szczotka, O.D., M.S. (principal investigator); Beth Ann Benetz, M.A.; Ellen Burnside; Stephanie Burke; Janet Edgerton, C.O.T.; Patricia Kane; Jonathan Lass, M.D.; Kimberly Schach; Stephanie Shaffer, M.A.; Pamela Smith; Thomas Stokkermans, O.D. Ph.D.
Gundersen Lutheran, La Crosse, Wis.: John Sterling, O.D. (principal investigator); Thomas Edwards, O.D.; Janet Hess; John Larson, O.D.; Jill Nelson; Lorna Plenge; John Sake; Eric Sheahan.
University of Illinois at Chicago Department of Ophthalmology: Timothy McMahon, O.D. (principal investigator); S. Barry Eiden, O.D.; Charlotte Joslin, O.D.; Tina Laureano; George Rosas.
Indiana University School of Optometry, Bloomington, Ind., and Indianapolis Eye Care Center: Gerald Lowther, O.D. Ph.D. (principal investigator); Carolyn Begley, O.D., M.S.; Donna Carter; Nikole Himebaugh, O.D.; Pete Kollbaum, O.D.; Colleen Riley, O.D. M.S.; Lee Wagoner, M.H.A.
Jules Stein Eye Institute, UCLA, Los Angeles: Barry Weissman, O.D. Ph.D. (principal investigator); Lilian Andaya; Lisa Barnhart, O.D.; Melissa Chun, O.D.; Ronit Englanoff, O.D.; Elisabeth Lim; Louis Rosenberg, O.D.; Karen Yeung, O.D.
University of Missouri-St. Louis School of Optometry: Larry Davis, O.D. (principal investigator); Edward Bennett, O.D. M.S.Ed; Zansheree Blue; Beth Henderson, O.D.; Bruce Morgan, O.D.
State University of New York State College of Optometry, New York: David Libassi, O.D. (principal investigator); Ralph Gundel, O.D.
Northeastern Eye Institute, Scranton, Pa.: Joseph Shovlin, O.D. (principal investigator); John Boyle, O.D.; Bradley Flickinger, O.D.; Elizabeth Flickinger, O.D.; Stephen Gushue; Patricia McMasters; Stephen Pascucci, M.D.
Nova Southeastern University College of Optometry, Ft. Lauderdale, Fla.: Heidi Wagner, O.D. (principal investigator); Andrea M. Janoff, O.D.; Chris Woodruff, O.D.; Arnie Patrick, O.D.; Julie A. Tyler, O.D.
Ohio State University College of Optometry, Columbus, Ohio: Barbara Fink, O.D. Ph.D. (principal investigator); Gregory Nixon, O.D.; Jason Nichols, O.D. M.S.
Pennsylvania College of Optometry, Elkins Park, Pa.: Joel Silbert, O.D. (principal investigator); KennethDaniels, O.D.; Mary Jameson; Theresa Sanogo.
Southern California College of Optometry, Fullerton, Calif.: Julie Yu, O.D., (principal investigator); Timothy Edrington, O.D. M.S.; Eunice Myung, O.D.; Julie Schornack, O.D. M.Ed.
University of Utah, John Moran Eye Center, Department of Ophthalmology, Salt Lake City: Harold Olafsson, O.D. (principal investigator); Doug Blanchard; Deborah Harrison, M.S.; Mark McKay, O.D.; Paula Morris; Kim Wegner.
Chairmans Office, Ohio State University College of Optometry: Karla Zadnik, O.D. Ph.D. (chairman); Nora McFadden (secretary); Jodi Malone, R.N. (study coordinator); Jeffrey Walline, O.D., M.S.
CLEK Photography Reading Center, Ohio State University College of Optometry: Joseph T. Barr, O.D., M.S. (director); Gilbert E. Pierce, O.D. Ph.D.; Marjorie J. Rah, O.D. Ph.D. (reader, New England College of Optometry); Mohinder Merchea, O.D., M.S.; Beth Ann Oglevee; Gloria Scott-Tibbs.
Coordinating Center, Washington University Medical School, Department of Ophthalmology and Visual Sciences, and Division of Biostatistics, St. Louis: Mae Gordon, Ph.D. (director); Joel Achtenberg, M.S.W; Patricia Nugent; Teresa Roediger; Kenneth Schechtman, Ph.D.; Brad Wilson, M.A.
CLEK Topography Reading Center, Department of Ophthalmology and Visual Sciences, University of Illinois at Chicago: Dr. McMahon (director); Robert Anderson, Ph.D.; Cynthia Roberts, Ph.D.; Michi Goto; Thomas Raasch, O.D. Ph.D.; George Rosas; Stephanie Schoepfer-Grosskurth; Dr. Szczotka; Mark Wright, M.S.
Project Office, National Eye Institute, Rockville, Md.: Donald F. Everett, M.A.
Executive Committee: Dr. Zadnik (chair); Drs. Barr, Gordon, Edrington and McMahon; Mr. Everett.
CLEK Topography Analysis Group: Drs. Szczotka and McMahon (co-chairs); Drs. Anderson, Friedman, Davis and Raasch.
Data Monitoring and Oversight Committee: Gary Cutter, Ph.D. (chair); Robin Chalmers, O.D.; Bruce Barron, M.D.
1. Zadnik K, Barr JT, Edrington TB, Everett DF, Jameson M, McMahon TT, Shin JA, Sterling JL, Wagner H, Gordon MO, Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Invest Ophthalmol Vis Sci 1998;39:2537-46.
2. Davis LJ, Schechtman KB, Begley CG, Shin JA, Zadnik K, CLEK Study Group. Repeatability of refraction and corrected visual acuity in keratoconus, Optom Vis Sci 1998;75:887-96.
3. Edrington TB, Szczotka LB, Barr JT, Achtenberg JF, Burger DS, Janoff AM, Olafsson HE, Chun MW, Boyle JW, Gordon MO, Zadnik K, Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Rigid contact lens fitting relationships in keratoconus. Optom Vis Sci 1999;76:692-9.
4. Gundel RE, Libassi DP, Zadnik K, Barr JT, Davis L, McMahon TT, Edrington TB, Gordon MO. Feasibility of fitting contact lenses with apical clearance in keratoconus. Optom Vis Sci 1996;73:729-32.
5. Barr JT, Zadnik K, Wilson BS, Edrington TB, Everett DF, Fink BA, Shovlin JP, Weissman BA, Siegmund K, Gordon MO, CLEK Study Group. Factors associated with corneal scarring in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Cornea 2000;19:501-7.

