Ocular and periocular infections present in a variety of locations from a variety of causes. The appropriate treatment course varies from observation to non-pharmacologic measures to the prescription of topical or oral anti-infective agents.1-3 In the absence of the need for a bacterial culture, which is generally reserved for vision-threatening conditions, a careful history and thorough examination guides the clinician to determine the most likely underlying cause that can inform treatment.
To muddy the waters further, narrowing down the diagnosis to suspecting a bacterial etiology leaves the clinician with a broad range of treatment strategies. Known patterns of resistance, allergy history and systemic history complicate the choice between antibiotics in clinical practice. While treatment failure may be caused by inaccurate diagnosis, the cause may also be inadequate dosing, organism resistance, development of toxicity or allergy or nonadherence.
The chosen route of treatment typically depends on the tissue involved: soft tissue infections such as preseptal cellulitis and dacyrocystitis require antimicrobial drug levels at the site of infection, which typically requires oral administration. Conjunctival and corneal disease is most commonly treated efficiently with topical agents, while externally pointed hordeola may respond well to topical ointments and hot compresses.1-3
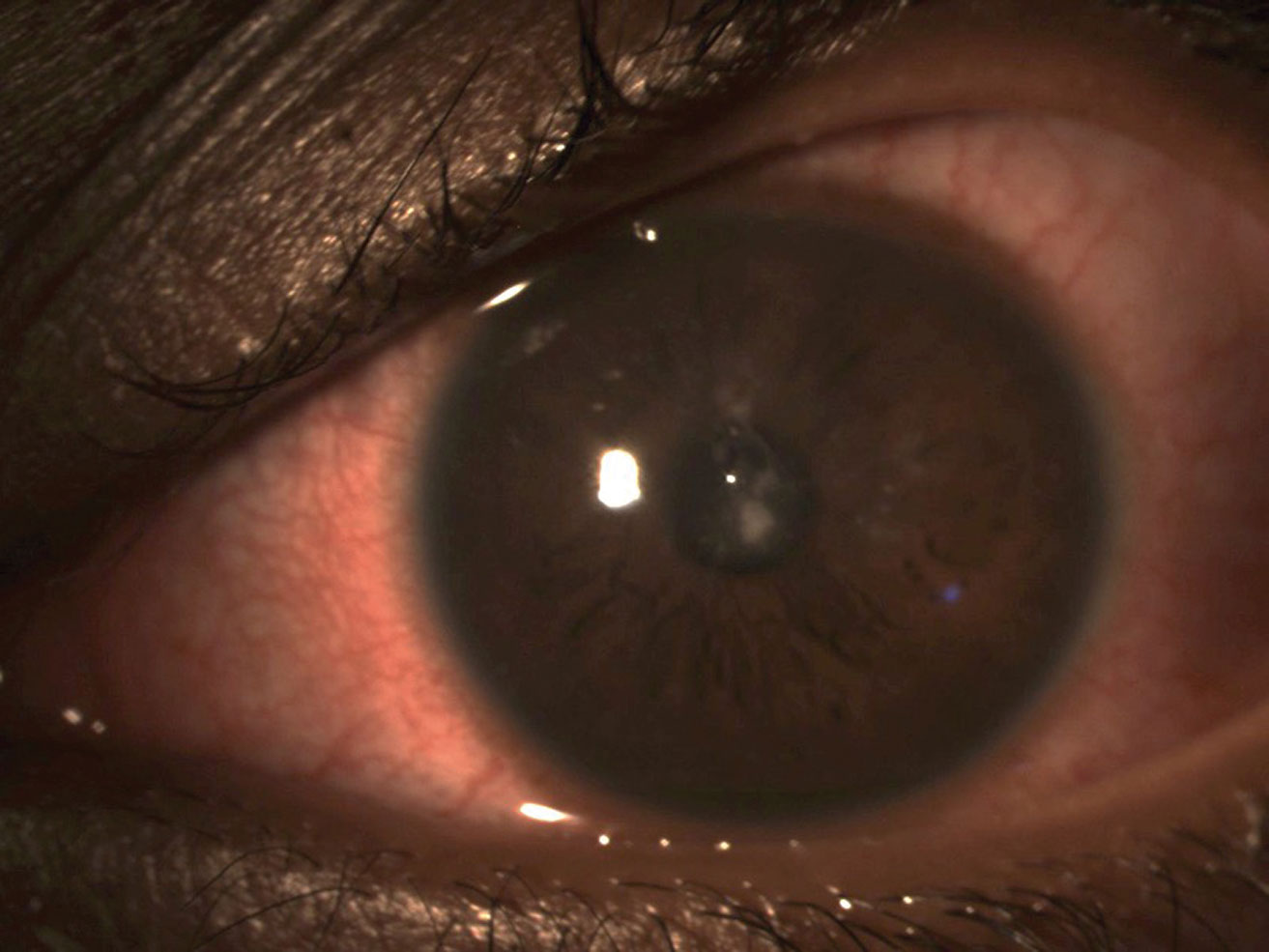 |
| Bacterial keratitis in a soft contact lens wearer. Click image to enlarge. |
The Dangers of Overtreatment
While most anti-infective agents used in periocular infection management are considered to be broad-spectrum, emerging trends in pathogen resistance have resulted in the need for understanding most common underlying pathogens likely to cause infection and their susceptibility to commonly prescribed antibiotics. However, overtreatment through unnecessary prescribing as well as undertreatment of antibiotics persists.
Up to 80% of uncomplicated acute conjunctivitis in adults are viral in nature. In those where bacterial infection is the underlying cause, cases are typically self-limiting within seven days. Considering their self-limiting nature, topical antibiotics may not be prescribed; however, they modestly improve (by approximately two days) the time to resolution compared with supportive therapy alone.1 Interestingly, nearly 60% of patients with acute conjunctivitis in the United States fill prescriptions for topical antibiotics.4
The gap between the number of cases of conjunctivitis that may benefit from topical antibiotic therapy and the number of prescriptions filled highlights the concern of over-prescribing antibiotics when not clinically indicated, a driver of antibiotic resistance.
The concept of a pre-determined set course of antibiotic therapy as a broad prescribing strategy aims to ensure complete eradication of disease-causing bacteria.5 But a one-size-fits-all treatment approach ignores a central component to patient care: the patient. The fear of undertreatment has led prescribers to generally prescribe the same duration of treatment regardless of the patient’s response to therapy.5
If our goal of appropriate antibiotic prescribing is centered on limiting antibiotic use based on necessity and minimizing side effects to reach clinical efficacy, antibiotic therapy discontinuation should differ based on the patient’s response and how follow-up care goes. One should only continue treatment with topical ocular antibiotics for the prophylaxis of infection—for example, in cases of corneal abrasion, while the increased risk is present—while epithelial damage exists rather than for a pre-determined course.
The minimum inhibitory concentration (MIC) value describes the in vitro susceptibility or resistance levels of bacteria to an antibiotic and serves as the basis for assessing whether an antibiotic may be effective in a clinical setting. Undertreatment of an infection can cause failure to reach the MIC, resulting in bacteria survival, mutation and replication during treatment, increasing the likelihood of development of a drug-resistant population.6 Never taper antibiotic agents below their equivalent MIC, which is often clinically represented by FDA-approved dosing. In order to taper a steroid in a topical antibiotic and steroid combination, first switch the patient to a steroid agent alone, rather than taper the combination agent.
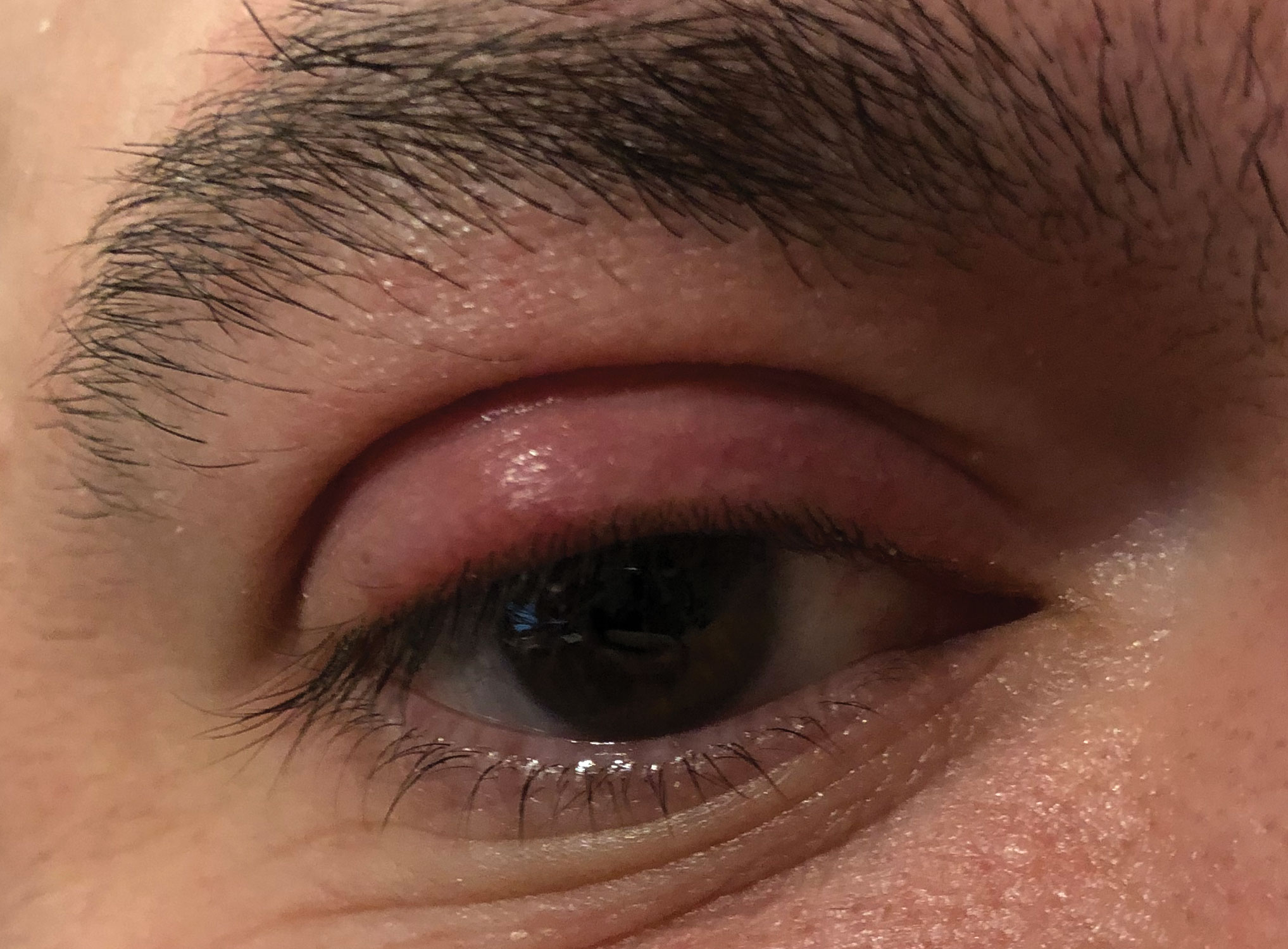 |
| This internally pointed hordeolum of the upper eyelid may respond well to topical ointments and hot compresses. Photo: Greg Caldwell, OD. Click image to enlarge. |
A Better Understanding
The main source of bacteria causing periorbital soft tissue infection or conjunctivitis in adults is the natural flora of the eyelids and conjunctiva. Common pathogens include gram-positive cocci, such as Staphylococcus epidermidis, Staphylococcus aureus and streptococcus species as well as Proteus miribalis, Serratia marcenas and gram-negative Pseudomonas species.2.3,7,8 In children, Haemophilus influenzae and Streptococcus pneumoniae are the most common ocular isolates in conjunctivitis cases.8,9
A basic understanding of the trends of resistance of common ocular isolates forms the basis for antibiotic selection for treatment with topical ocular antibiotics and oral medications to treat periocular infections. The Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) study surveyed antibiotic resistance patterns of common ocular isolates.8 Its 10-year results showed that while there was no significant increase in the rates of resistance during the study period, there was generally increased resistance with age.8 A significant proportion of S. aureus isolates were resistant to oxacillin/methicillin (MRSA) and at least three classes of antibiotics, termed to be “multi-drug resistant.” Many believe resistance and susceptibility patterns to closely mirror patterns in systemic infections, so we can apply these findings to treatment of ocular infections with systemic and topical antibiotics.8
The study also found that more than half of all S. aureus isolates were resistant to azithromycin, and 33.3% were resistant to ciprofloxacin. MRSA isolates were nearly all resistant to azithromycin. Importantly, MRSA isolates were determined to be highly susceptible to trimethoprim and tetracycline, but a high percentage of isolates displayed resistance to fluoroquinolones other than besifloxacin.8 S. pneumoniae isolates were most likely to be resistant to azithromycin (36.3%) and penicillin (32.2%). P. aureginosa isolates displayed the lowest MIC with ciprofloxacin.8
In general, azithromycin shows widespread resistance to the common ocular isolates implicated in ocular and periocular disease, including MRSA, which is likely a result of the widespread use of the medication. Specific resistance strategies of macrolides may be compounded by the medication’s long half-life and make azithromycin a non-preferred choice for the treatment of soft tissue infections or bacterial conjunctivitis.8,10
Pertinent to infections in children, nearly all H. influenzae isolates were susceptible to all antibiotics evaluated including ofloxacin, ciprofloxacin, moxifloxacin, azithromycin and tetracycline.8
The ARMOR trial also determined resistance to besifloxacin to be negligible. Besifloxacin has no systemic formulation, and due to its mechanism of action, the development of resistance requires mutation in both DNA gyrase and DNA topoisomerase IV.8
While community-acquired rates of MRSA have increased over time, individuals in healthcare settings, those recently released from the hospital, incarcerated patients and patients with a history of MRSA carry a greater possibility of harboring MRSA and therefore developing a bacterial infection due to MRSA.3 Based on the ARMOR data on treating bacterial infections in patients who may be at increased risk of MRSA, a topical agent containing trimethoprim, such as trimethoprim sulfate and polymyxin B sulfate or besifloxacin, is preferred.8 For the treatment of infections requiring oral medication, consider trimethoprim 160mg and sulfamethaxazole 800mg, or for sulfonamide allergic patients, doxycycline 100mg.
For the treatment of bacterial conjunctivitis in children, where the gram-negative bacteria H. influenzae is a common underlying cause, consider fluoroquinolones such as ciprofloxacin 0.3%, moxifloxacin 0.5% and ofloxacin 0.3% ophthalmic solution. The safety and efficacy of these medications have been evaluated in children one year of age and older.11-13
For children younger than one year old, researchers have evaluated polymyxin B sulfate and trimethoprim sulfate in children as young as two months. While trimethoprim is not effective against H. influenzae, polymyxin B can provide coverage against this specific organism.14
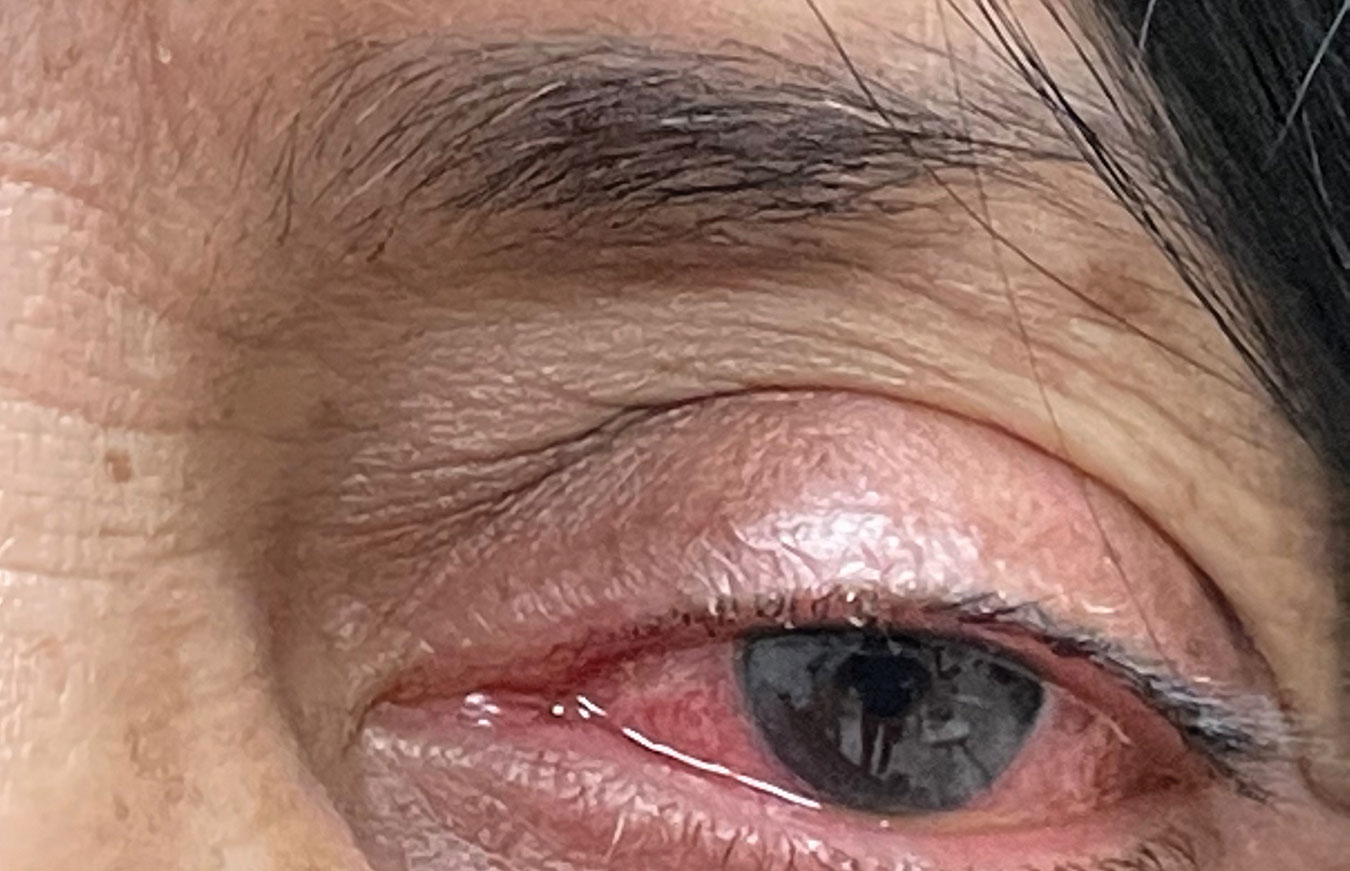 |
| Hypersensitivity reaction of the left eye secondary to topical brimonidine use. Differentiating between a true type-1 hypersensitivity reaction or an IgE-mediated response and another type of adverse effect requires a careful discussion of symptoms the patient experienced with each reported adverse event. Click image to enlarge. |
Pregnancy Considerations
Every prescribing decision is based on a careful understanding and discussion with the patient of the risks, benefits and expected result of treatment. The lack of data regarding prescription medication safety in pregnant individuals stems from protections implemented to limit the study of pregnant individuals, which resulted in the difficulty of inclusion of pregnant individuals in clinical trials. This resulting lack of data may make both the prescriber and the patient reluctant to treat the underlying condition.15
Taking medications during pregnancy or during lactation is common, with an estimated 90% of individuals taking an over-the-counter or prescription medication during pregnancy, which means that 5.4 million pregnancies are exposed to medications annually.16 When treating pregnant individuals, make note of alcohol use, drug use and smoking as well as inappropriately prescribed medications. Consider the risks and benefits of therapy for the patient and fetus.17
In general, penicillins and cephalosporins are safe to use during pregnancy.18 Of the macrolides, azithromycin is generally safe to use, while one should use erythromycin and clarithromycin with caution and only when the benefit of treatment outweighs the risk.18 Tetracyclines have proven teratogenicity in humans, and fluoroquinolones have concerns of fetal malformation in animals and therefore should be avoided. Avoid trimethoprim and sulfamethoxazole in the first trimester of pregnancy and after 32 weeks of gestation.18 While systemic absorption with concurrent punctal occlusion of any topical ophthalmic agent is low, many consider topical ophthalmic antibiotic agents to be safe for use in pregnant individuals. Still, take additional clinical risk-benefit consideration for topical ophthalmic fluoroquinolones due to the risk that systemically applied agents pose.
Adverse Effects Review
When discussing the risks, benefits and expected effects of a prescribed oral antibiotic with patients, do devote a part of the conversation to likely and possible side effects. Due to orally administered antibiotics’ disruption of disease-causing flora and natural gastrointestinal flora, antibiotic-associated diarrhea is common and can be expected in approximately 30% of individuals—one of the most common reasons for nonadherence to antibiotic treatment.19
Probiotics are ingested microorganisms, including Lactobacillus, Bifidobacterium and others, that may help to maintain or restore the natural gastrointestinal flora and ecosystem during and after antibiotic treatment; however, not all patients will respond favorably, with a determined number of 13 individual species that need to be treated in order to improve one case of antibiotic-associated diarrhea.20
While reconstitution of the natural gut flora is often slow following antibiotic treatment, probiotics may further delay the reconstitution to a natural gastrointestinal microbiome. Notably, gastrointestinal flora is unique to individuals. By ingesting a large amount of specific probiotic organisms, which are often not an exact reflection of the composition of an individuals’s flora, the introduced species may dominate and delay recolonization of natural flora by up to five months, compared with individuals who did not use probiotics.21
While warnings about taking antibiotics with alcohol are often added to prescription bottles, the evidence for reduced efficacy for most antibiotics is lacking. Erythromycin, which may be prescribed in the context of periocular disease treatment, should not be taken with alcohol due to the potential for reduced efficacy and potential systemic toxicity.22
Also, cephalosporins and sulfonamide antibiotics carry a specific adverse effect that patients should be advised of. A “disulfiram-like” reaction describes specific symptoms that mimic the effect of disulfiram, a medication used for the treatment of alcohol dependency disorder. Patients may experience dizziness, sweating, headache, nausea, weakness, vomiting and hypotension—symptoms often described to be similar to the exacerbated effects of a hangover. While disulfiram-like reactions are well-documented with cephalosporin and alcohol use, only isolated case reports have been described following the administration of trimethoprim-sulfamethaxazole.23,24
While azithromycin is a commonly prescribed oral antibiotic, recent reports note an increased risk of sudden cardiac death due to prolongation of the QT interval in individuals with cardiovascular event risk factors that later prompted the FDA to include a black box warning for the drug.25 Individuals most at risk are those with underlying arrhythmia termed torsades de pointes.25
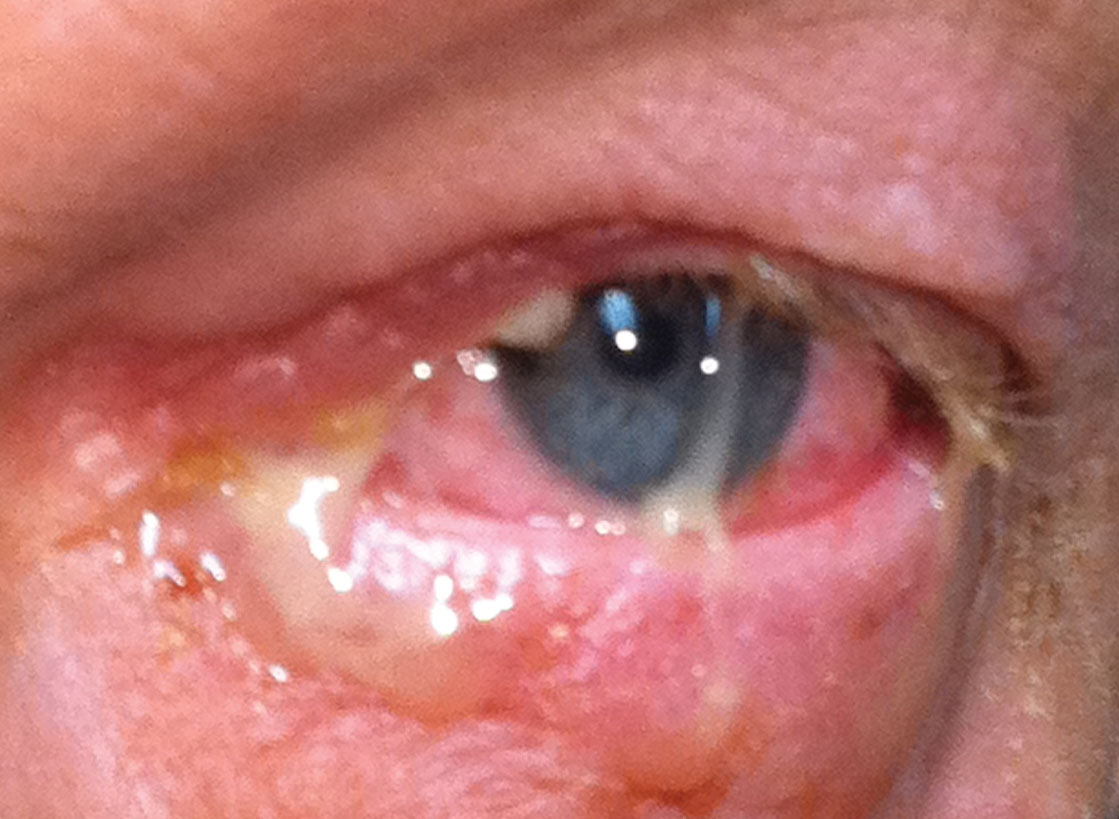 |
| Mucopurulent discharge characteristic of bacterial conjunctivitis. Photo: Greg Caldwell, OD. Click image to enlarge. |
Allergy Perils
Prior to prescribing any medication, perform a careful review of history of adverse drug reactions and allergies. Differentiating between a true type-1 hypersensitivity reaction or an IgE-mediated response and another type of adverse effect requires a careful discussion of symptoms the patient experienced with each reported adverse event.
Urticaria, anaphylaxis, bronchospasm, laryngospasm and angioedema are all signs of true allergy, while gastrointestinal upset is a sign of an expected adverse effect of orally administered antibiotics.26,27 While approximately 10% of the population have reported an allergy to penicillin, less than 5% have a true allergy.26 The allergic potential of sulfonamide antibiotics carries a similar risk, with approximately 3% of the population having experienced a true allergic reaction.28
The practice of avoiding cephalosporins in penicillin-allergic patients stems from the potential for cross-reactivity due to their structurally similar R1 side-chain of first- (i.e., cephalexin) and second-generation cephalosporins. The risk of allergy to a cephalosporin in a penicillin-allergic patient due to cross-reactivity is much less than the historically understood 10%.29 However, avoid cephalexin in patients with known penicillin hypersensitivity due to the approximate 1% cross-reactivity with first-generation cephalosporins.29
When patients have a true allergy to one type of drug—for instance, to a sulfonamide non-antibiotic such as a thiazide diuretic—is there potential for allergy to a structurally similar drug class, such as a sulfonamide antibiotic? While the potential exists for allergic reaction in both classes, it appears to not be due to structural similarity and development of cross-reactivity but rather increased propensity to allergic reaction in general.
Pay particular attention to patients with an atopic history or pre-existing asthma diagnosis, especially when they have a history of penicillin or sulfonamide antibiotic allergy. Either of these can suggest that multiple allergies may exist across non-structurally similar drug categories.27
Takeaways
Optimal prescribing for the treatment of bacterial infections requires careful clinical examination, a thorough history and an understanding of the microbiome of periocular tissues, resistance and susceptibility patterns and risk of potential adverse effects. Using the right antibiotic at the right time, dose and duration will ensure that your patient is appropriately managed while minimizing the risk of adverse effect to your patient and the greater community.
Dr. Steen is an attending optometrist and assistant professor of ocular pharmacology at Nova Southeastern University College of Optometry. She has no financial interests to disclose.
| 1. Azari AA, Barney NP. Conjunctivitis: a systematic review of diagnosis and treatment. JAMA. 2013; 310(16):1721-9. 2. Mannis MJ, Plotnick RD. Bacterial conjunctivitis. In: Tasman W, Jaeger EA, ed. Duane’s Clinical Ophthalmology. Vol. 4. Philadelphia: Lipencott-Raven; 2004:1-7. 3. Charalampidou S, Connell P, Fennell J, et al. Preseptal cellulitis caused by community acquired methicillin resistant Staphylococcus aureus (CAMRSA). Br J Ophthalmol. 2007;91(12):1723-4. 4. Shekhawat NS, Shtein RM, Blachley TS, et al. Antibiotic prescription fills for acute conjunctivitis along enrollees in a large United States managed care network. Ophthalmology. 2017;124(8):1099-1107. 5. Llewelyn MJ, Fitzpatrick JM, Darwin E, et al. The antibiotic course has had its day. BMJ. 2017;358;j3418. 6. Kowalska-Krochmal B, Dudek-Wicher R. The minimum inhibitory concentration of antibiotics: methods, interpretation, clinical relevance. Pathogens. 2021;10(2):165. 7. Ratnumnoi R, Keorochana N, Sontisomabat C. Normal flora of conjunctiva and lid margin, as well as its antibiotic sensitivity, in patients undergoing cataract surgery at Phramongkutklao Hospital. Clin Ophthalmol. 2017;11:237-41. 8. Asbell PA, Sanfilippo CM, Sahm DF, et al. Trends in antibiotic resistance among ocular microorganisms in the United States from 2009 to 2018. JAMA Ophthalmol. 2020;138(5):439-50. 9. Cavuoto K, Zutshi D, Karp CL, et al. Update on bacterial conjunctivitis in South Florida. Ophthalmology. 2008;115(1):51-6. 10. Food and Drug Administration. Highlights of prescribing information: Zithromax. 2019. www.accessdata.fda.gov/drugsatfda_docs/label/2019/050710s049,050711s047,050784s034lbl.pdf. Accessed April 9, 2021. 11. Novartis Pharma AG. Prescribing information: Ciloxan. 2013. www.novartis.com.sg/system/files/product-info/CILOXAN%20STERILE%20OPHTHALMIC%20AND%20OTIC%20SOLUTION%200.3%25.pdf. Accessed April 9, 2021. 12. Food and Drug Administration. Highlights of prescribing information: Vigamox. 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/21598s024lbl.pdf. Accessed April 9, 2021. 13. Allergan. Prescribing Information: Ocuflox. 2016. www.accessdata.fda.gov/drugsatfda_docs/label/2016/019921s021lbl.pdf. Accessed April 9, 2021. 14. Allergan. Prescribing Information: Polytrim. 2018. media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/20180622-POLYTRIM-USPI-1-0USPI7824.pdf. Accessed April 9, 2021. 15. van der Graaf R, van der Zande ISE, den Ruijter HM, et al. Fair inclusion of pregnant women in clinical trials: an integrated scientific and ethical approach. Trials. 2018;19(1):78. 16. Mitchell AA, Gilboa SM, Werler MM, et al. National birth defects prevention study. Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obstet Gynecol. 2011;205(1):51.e1-8. 17. Genetic Alliance; District of Columbia Department of Health. Understanding genetics: a District of Columbia guide for patients and health professionals. Washington (DC): Genetic Alliance; 2010 Feb 17. Appendix D, Teratogens/Prenatal Substance Abuse. 18. Lamont HF, Blogg HJ, Lamont RF. Safety of antimicrobial treatment during pregnancy: a current review of resistance, immunomodulation and teratogenicity. Expert Opin Drug Saf. 2014;13(12):1569-81. 19. Szajewska H, Mrukowicz JZ. Probiotics in prevention of antibiotic-associated diarrhea:meta-analysis. J Pediatr. 2003;142(1):85. 20. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307(18):1959-69. 21. Suez J, Zmora N, Zilberman-Schapira G, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174(6):1406-23.e16. 22. Mergenhagen KA, Wattengel BA, Skelly MK, et al. Fact versus fiction: a review of the evidence behind alcohol and antibiotic reactions. Antimicrob Agents Chemother. 2020;64(3):e02167-19. 23. Barth KS, Malcolm RJ. Disulfiram: an old therapeutic with new applications. CNS Neurol Disord Drug Targets. 2010;9(1):5-12. 24. Heelon MW, White M. Disulfiram-cotrimoxazole reaction. Pharmacotherapy. 1998;18(4):869-70. 25. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012; 366(20):1881-90. 26. Shenoy ES, Macy E, Rowe T, et al. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321(2):188-99. 27. Strom BJ, Schinnar R, Apter AJ, et al. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N Engl J Med. 2003;349(17):1628-35. 28. Lieberman P, Anderson JA, eds. Allergic diseases: diagnosis and treatment. Totowa, NJ:Humana Press; 1997:289. 29. Campagna JD, Bond MC, Schabelman E, et al. The use of cephalosporins in penicillin-allergic patients: a literature review. J Emerg Med. 2012;42(5):612-20. |

