 |  |
In the last several columns, we have discussed the many innovative procedures now being used to treat corneal endothelial disease. Below, we’ll summarize the surgical options we have previously covered and provide an overview of where we’re at and what’s ahead in corneal endothelial disease therapy.
The Evolution
Traditionally, corneal transplants were accomplished exclusively by full-thickness penetrating keratoplasty. However, this procedure often results in suboptimal visual outcomes and carries inherent risks of long-term complications such as graft rejection or perforation after minor ocular trauma.
The need for new and improved surgical techniques eventually led to endothelial keratoplasty, allowing the surgeon to manipulate only posterior corneal layers while leaving healthy anterior tissue intact. This procedure has significantly improved visual outcomes compared to PK but can still be complicated by graft rejection or dehiscence.
Management of corneal endothelial diseases continues to evolve. Over the last few years, a new technique to treat Fuchs’ dystrophy has emerged. Known as Descemet stripping only (DSO), this procedure involves stripping the central 4mm to 5mm of Descemet’s membrane, resulting in peripheral endothelial cell migration to fill in the stripped areas.1 DSO is only indicated in eyes with guttae centrally but viable endothelium peripherally, and the cornea must have a peripheral endothelial cell count of at least 1,000 cells/mm2 with no other corneal pathology.2 Compared to Descemet stripping endothelial keratoplasty (DSEK) or Descemet’s membrane endothelial keratoplasty (DMEK), DSO is faster, easier and cheaper, eliminates complications of long-term steroid use and negates graft-related complications such as rebubbling, failure and rejection.3 Topical rho-kinase inhibitors have been found to improve corneal clearance in conjunction with this procedure.4
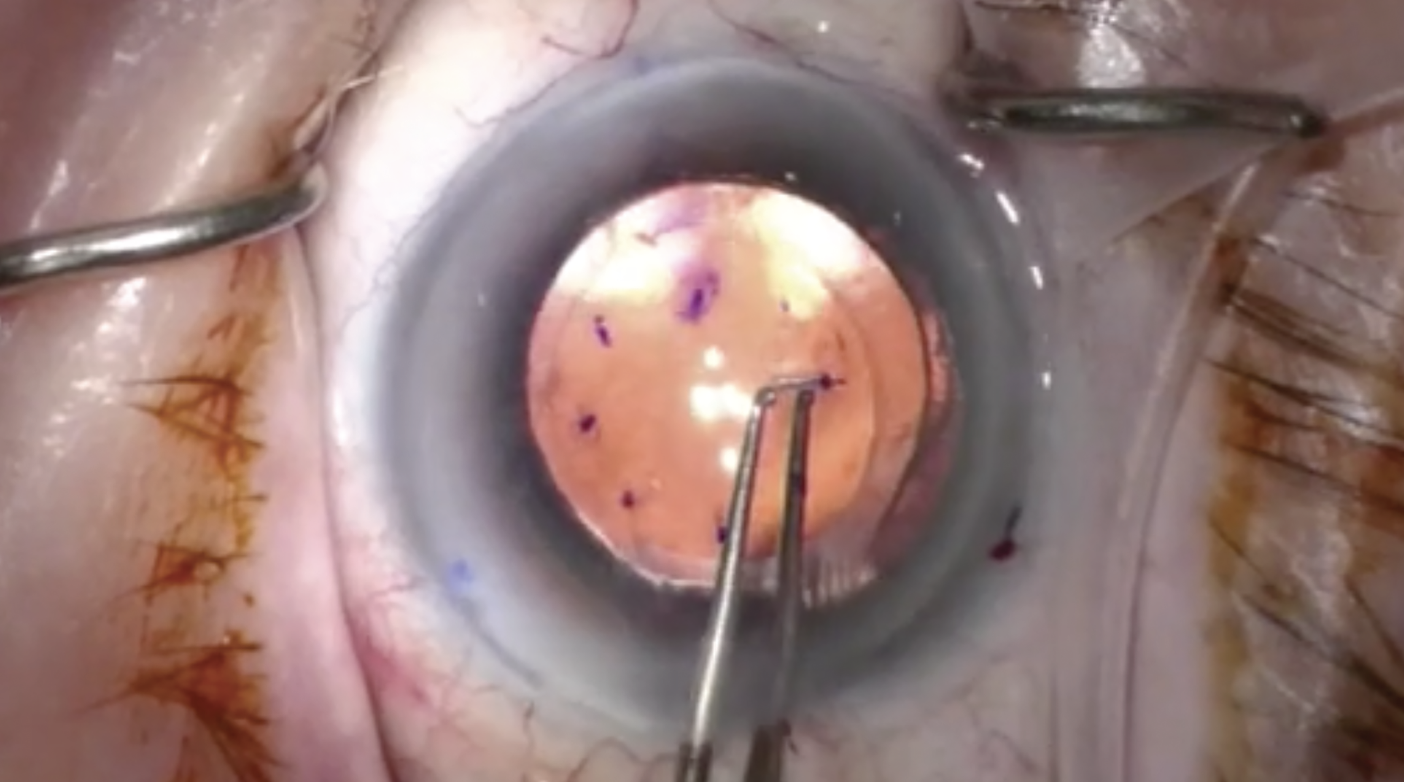 |
| DSO has several advantages over DSEK or DMEK; it’s faster, easier, cheaper, eliminates complications associated with prolonged steroid use and reduces graft-related issues such as rebubbling, graft failure and rejection. Click image to enlarge. |
Investigative Treatments
Nonsurgical innovations are also being investigated. Emmecell, a biotech company, is investigating a minimally invasive approach using a magnetic cell delivery nanoparticle platform.5 Donor endothelial cells are isolated and combined with magnetic nanoparticles to form magnetized human corneal endothelial cells, which are injected into the anterior chamber. The patient then wears an external magnetic eye patch to help form an endothelial scaffold on the posterior corneal surface. Phase I data shows the treatment is well tolerated.6
Another ongoing Phase I/II trial, by Aurion Biotech, is investigating a combination product of human corneal endothelial cells and a rho-kinase inhibitor, which may replace and repair the endothelial architecture and function.7 In March 2023, this product was approved in Japan, where the first clinical trials were performed. In those, 11 patients with significant endothelial cell loss received a single injection of cell therapy into the anterior chamber and laid face down for three hours post-op.
At the two-year follow-up, all corneas were clear and showed a decrease in corneal thickness.8 After five years, corneal endothelial function was restored in 10 of the 11 eyes.9 This may help resolve worldwide donor tissue shortages and greatly reduce the need for surgical transplantations.
Internationally, preliminary investigation of new technology is also taking place. EyeYon, an Israeli-based company, is investigating the EndoArt, a 50µm-thin artificial endothelial layer implant used as an alternative to a DMEK graft to treat chronic corneal edema. The device is surgically inserted similarly to a DMEK graft and functions by creating a barrier for aqueous fluid to enter the cornea. In theory, this would eliminate the complications associated with graft rejection, as donor tissue is not required. Early results appear promising.10
These promising innovations may greatly reduce the need for traditional corneal transplantation surgery. As new technology becomes available, the management of corneal edema may largely shift from surgical to medical treatment options.
Dr. Cunningham is the director of optometry at Dell Laser Consultants in Austin, TX. He has no financial interests to disclose. Dr. Whitley is the director of professional relations and residency program supervisor at Virginia Eye Consultants in Norfolk, VA. He is a consultant for Alcon.
Dr. Black graduated from Nova Southeastern College of Optometry and completed residency in ocular disease at Bascom Palmer Eye Institute. He practices at Virginia Eye Consultants in Virginia Beach. He has no financial disclosures.
1. Borkar DS, Veldman P, Colby KA. Treatment of Fuchs endothelial dystrophy by Descemet stripping without endothelial keratoplasty. Cornea. 2016;35(10):1267-73. 2. Mannis MJ. Cornea. 5th ed. Elsevier; 2022. 3. Huang MJ, Kane S, Dhaliwal DK. Descemetorhexis without endothelial keratoplasty versus DMEK for treatment of Fuchs endothelial corneal dystrophy. Cornea. 2018;37:1479-83. 4. Moloney G, Petsoglou C, Ball M, et al. Descemetorhexis without grafting for Fuchs endothelial dystrophy-supplementation with topical ripasudil. Cornea. 2017;36(6):642-8. 5. Study of safety and tolerability of EO2002 in the treatment of corneal edema. clinicaltrials.gov/study/NCT04894110. 6. Kunzevitzky N, Fleming C, Thoele JK, et al. Phase 1 multicenter study of magnetic cell therapy for corneal edema. Invest Ophthalmol Vis Sci. 2022;63(7):2758-A0247. 7. A phase 1/2 study of AURN001 in subjects with corneal edema secondary to corneal endothelial dysfunction (ABA-1) (CLARA). clinicaltrials.gov/study/NCT06041256. 8. Kinoshita S, Koizumi N, Ueno M, et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. N Engl J Med. 2018;378(11):995-1003. 9. Numa K, Imai K, Ueno M, et al. Five-year follow-up of first 11 patients undergoing injection of cultured corneal endothelial cells for corneal endothelial failure. Ophthalmology. 2021;128(4):504-14. 10. Auffarth GU, Son HS, Koch M, et al. Implantation of an artificial endothelial layer for treatment of chronic corneal edema. Cornea. 2021;40(12):1633-8. |

