Retina Takes the StageExperts offer pearls for detecting and managing numerous posterior segment conditions in the June 2024 issue of Review of Optometry, our 15th annual Retina Report. Check out the other articles featured in this issue: |
An estimated 15% to 30% of uveitis cases are of the posterior variety, making it the second most common form behind anterior uveitis. Following that, panuveitis is the third most common form of uveitis in western countries.2
The prevalence of uveitis has been estimated as high as 714 cases per 100,000 people.2 Uveitis, specifically posterior and panuveitis, can be visually debilitating, as it causes 10% to 15% of blindness in the world and is one of the leading causes of blindness in the United States.3 Panuveitis in particular has been correlated with a poorer visual prognosis. Given the prevalence of uveitis and the potential visual impact of posterior uveitis and panuveitis, it is important to understand characteristic presentations and etiologies for accurate diagnosis, which we will discuss extensively below.2
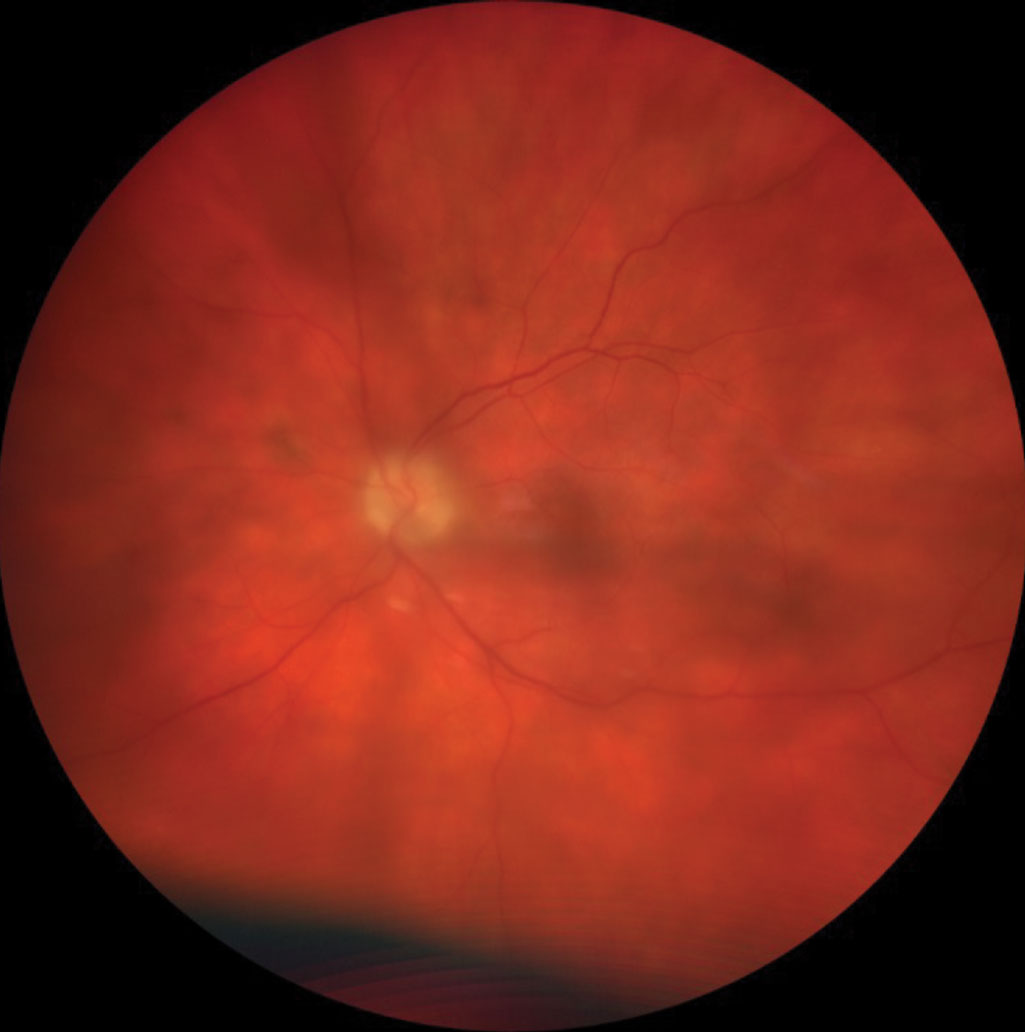 |
Patient with panuveitis demonstrating significant vitreal haze. Click image to enlarge. |
Creating an Accurate Differential Diagnosis
Forming an accurate differential list for posterior uveitis can be challenging. The most important step is taking a good history, as this condition can be caused by many different etiologies. Items to include are a complete medical history—including present and past personal medical history—surgeries, history of trauma, medicines and family history, along with demographics—age, sex and ethnicity. Other history items are arthritis, rashes, shortness of breath, swollen lymph nodes, recent headaches, hearing difficulties, hair loss, pigment changes in the skin, a history of ocular trauma, recent insect bites, sexually transmitted diseases, tuberculosis exposure, blood in stools and recent travel. It’s also important to include current history of present illness including onset, duration, laterality, course, associations and modifying factors.4Some patients have no symptoms with posterior uveitis; others present with acute floaters, loss of vision, photopsia and rarely pain. For panuveitis, floaters, loss of vision and photopsia can occur, along with symptoms of anterior uveitis including pain, hyperemia and light sensitivity.4,5
A thorough examination is also essential to forming differentials, including visual acuity, pupil testing, extraocular muscles, intraocular pressure, slit lamp examination and biomicroscopy. Determining which parts of the uveal tract have inflammation will also aid in the determination of your differential. Some disease processes will affect just the choroid (posterior uveitis) and others will affect the iris, ciliary body and choroid (panuveitis).5
Posterior uveitis presents with a myriad of potential clinical signs to identify: vitreal cells, flare and opacities, retinitis, vasculitis, peripheral periphlebitis, edema of the retina, macular or optic disc, retinal hemorrhages and vitreal hemorrhage. Complications of the inflammatory response include choroidal neovascular membranes, retinal detachment, retinal vascular occlusions, ischemia and retinal necrosis.
Panuveitis has similar signs and complications in the posterior aspect of the eye, but also affects the intermediate and anterior uveal tract. These signs include pars planitis, cells and flare in the anterior chamber and circumlimbal hyperemia.3-5
Many times, the signs you see during examination will guide your differentials. Consider the following questions:
1. Is it posterior uveitis only or is it part of a panuveitis?
2. Is it choroiditis, retinitis, chorioretinitis or retinochoroiditis?
3. Is there associated involvement of the optic nerve head and/or the retinal vessels?
4. Does the clinical feature fit into any known infective or non-infective entity?
5. Is there associated anterior segment inflammation, vitritis or complications?
6. Is it associated with other systemic features?
7. Is it recurrent? If so, how has it responded to previous therapy?
8. Is it associated with an immunocompromised state?
9. Is it a masquerade syndrome?6
Ancillary testing can also aid in the differential diagnosis of posterior uveitis. Fundus photography can be used to identify the extent of the inflammation and retinal changes. Fundus autofluorescence (FAF) can be useful in many cases of posterior uveitis, especially when evaluating white dot syndromes.
FAF patterns will vary depending on the type of white dot syndrome. Serpiginous choroidopathy shows large areas of hypoautofluorescence emanating from the optic nerve that are associated with atrophy in inactive lesions, whereas new lesions are associated with hyperautofluorescence. Another example of a unique pattern is found in acute zonal occult outer retinopathy with peripapillary hypoautofluorescence and a granular mixture of hyper- and hypoautofluorescence associated with active extension into the arcades.7,8
OCT is commonly used to identify changes in the posterior segment, including retinal, macular and optic disc edema, retinal atrophy, choroidal thickening and other changes.8,9 OCT-A can further help identify changes in blood vessel density and detection of choroidal neovascular membranes.9 Fluorescein angiography is used to detect and evaluate extent of retinal vasculitis, macular edema, papillitis and capillary nonperfusion. Ultrasounds are most useful when visualization of the fundus is obscured from vitritis as well as evaluating extent of exudative retinal and/or choroidal detachments when present. Indocyanine green angiography is an important imaging modality for disease that have a predominance for the choroid (e.g., Vogt-Koyanagi-Harada disease, birdshot chorioretinopathy).
Other systemic testing can be useful when identifying the cause of posterior uveitis, such as a chest X-ray, which should be considered when suspecting sarcoidosis or tuberculosis. If it’s sarcoidosis, bilateral hilar lymphadenopathy will show up and tuberculosis commonly shows lymphadenopathy, pleural effusion and caseating granulomas.10,11 MRIs can sometimes be useful when diagnosing neurosyphilis, as they will detect vasculitis of the small and middle cerebral arteries, infarction and hemorrhaging, although findings are not highly specific.12
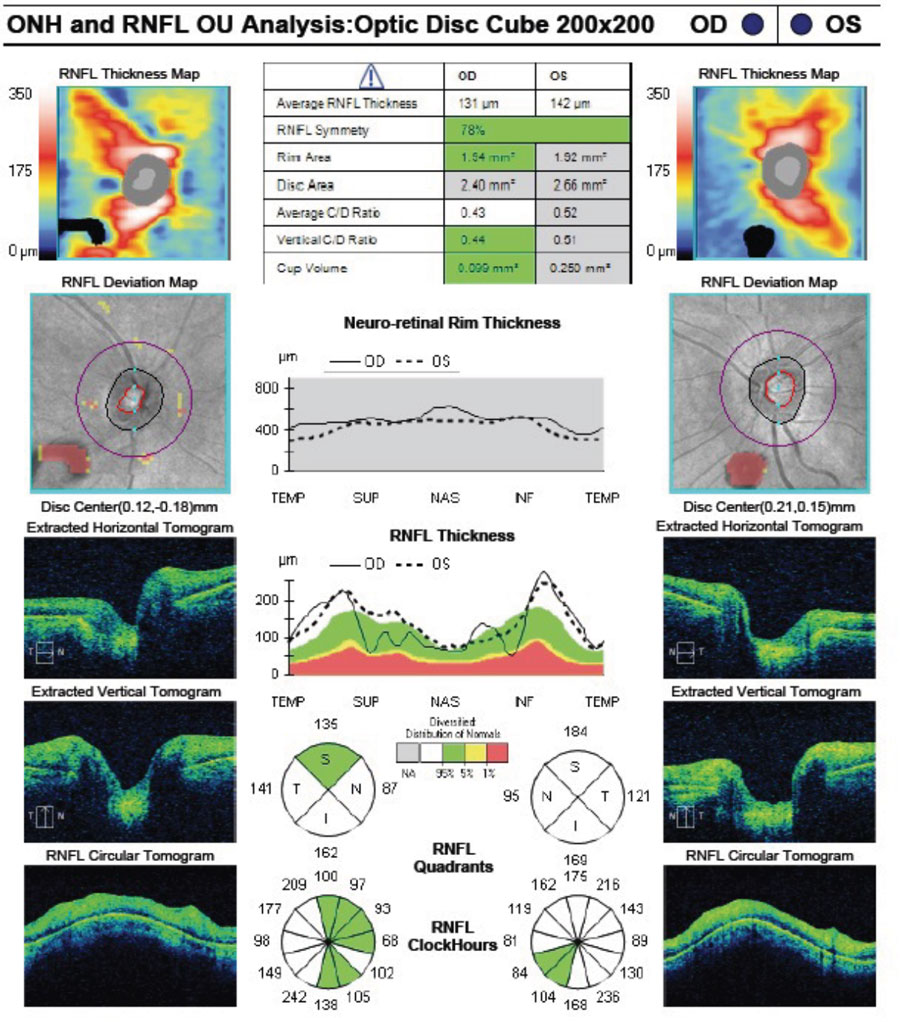 |
Significant bilateral macular edema secondary to bilateral panuveitis. Click image to enlarge. |
Etiologies
It is important to understand the underlying etiology of the disease process to guide treatment and referrals that may be necessary. There are specific and non-specific labs that should be considered when determining a diagnosis, and a proper examination will help narrow down the labs that should be ordered. In many cases, lab work will assist in confirming a diagnosis or ruling out important differential diagnoses.While uveitis can be idiopathic, posterior uveitis has a higher likelihood of a concomitant systemic medical condition. In over 75% of posterior uveitis cases, a specific diagnosis was found.13 Posterior and panuveitis have many different etiologies, the most common being infectious and inflammatory. They are usually associated with systemic medical conditions but can also be linked to primary ocular conditions. The most common etiologies and their typical presentations are divided into three general categories: infectious, inflammatory and ocular syndromes. We will cover the two most common categories, infectious and inflammatory, in more detail:
Infectious
It is always important to rule out infectious etiologies for posterior uveitis, and to know which infections to consider can help guide you in making an accurate diagnosis. A helpful mnemonic when coming up with differentials for infectious posterior uveitis is “STTEEVE”:14
S – Syphilis
T – Tuberculosis (TB)
T – Toxoplasmosis
E – Endogenous
E – Endophthalmitis
V – Viral (herpes simplex, herpes zoster, cytomegalovirus)
E – Etc. (e.g., Bartonella, toxocariasis)
Several bacterial infections cause posterior or panuveitis, but the most common are syphilis and TB. Syphilis is the result of an infection with the spirochete Treponema pallidum and can result in many systemic and/or ocular manifestations.15,16 Posterior uveitis presents in several different ways, including localized chorioretinitis or retinitis, which can be non-specific and mimic other diseases and is why syphilis is often called the “great masquerader.” Chorioretinal lesions are typically multifocal and bilateral and are associated with vitritis and/or exudative retinal detachment. Retinitis has been described as having a “ground glass” appearance and may be associated with vasculitis as well.15,16 Acute syphilitic posterior placoid chorioretinopathy is a more specific presentation of syphilitic posterior uveitis that appears most commonly as pale yellow, gray or white placoid lesions in the outer retina of varying size within the posterior pole.17 This lesion can best be visualized through FAF on which the placoid lesions hyperautofluoress.17
TB is a chronic bacterial infection caused by Mycobacterium tuberculosis. While acute infection is uncommon, many people can have latent TB and ocular TB can manifest without any systemic symptoms.18 Any part of the eye can be involved; however, uveitis is the most common ocular manifestation and occurs as either anterior, intermediate or posterior.18 Posterior uveitis in TB can have a wide range of manifestations, including retinitis, choroidal granulomas (e.g., tubercles and tuberculomas) and serpiginous choroiditis.14,18 Tubercles are the most recognizable ocular sign and appear as unilateral or bilateral, multiple (less than five), small, grayish white or yellow elevated lesions of the posterior pole with indistinct margins, lying deep within the choroid.18 Tuberculomas are larger and more often solitary with overlying hemorrhages and a more characteristic tumor-like appearance.14,18
Multifocal serpiginous choroiditis or serpiginous-like choroiditis, more common in TB endemic countries, present as multifocal lesions that are spread in a centrifugal serpiginous pattern sparing the peripapillary region and often accompanied by a vitritis.14,18
Toxoplasmosis, unlike syphilis and TB, is caused by a parasite, Toxoplasmosa gondii. It is the most common cause of infectious posterior uveitis in the world and classically presents as a unilateral focal necrotizing chorioretinitis with overlying vitritis.14,19 This presentation is often taught as having a “headlights-in-the-fog” appearance due to the hazy bright whiteish yellow lesion in the retina clouded by the overlying vitritis.19,20 It is common for an active lesion to be adjacent or close to an existing chorioretinal scar, suggesting reactivation of a previous infection. Infections often remains active for up to 16 weeks, leaving behind a hyperpigmented scar.14 Lesions are often found in the posterior pole and may even be accompanied by optic nerve edema. Macular scarring is one of the most common causes of vision loss secondary to toxoplasmosis posterior uveitis. A new infection without a pre-existing scar is relatively uncommon and typically only present in patients who are immunocompromised.20
Endogenous endophthalmitis is another potential infectious cause of posterior uveitis and panuevitis. The most common fungal culprits are Candida, Aspergillus and Coccidiomycosis, and present as creamy, white, discrete chorioretinal lesions of the posterior pole with overlying vitritis (fluffy cotton ball or string of pearls appearance).14 Endogenous bacterial endophthalmitis can be caused by a wide variety of bacteria.
Viral infections can lead to both posterior and panuveitis; however, anterior and intermediate uveitis are more common presentations. The most likely viral candidates for uveitis include herpes viruses: herpes simplex, varicella zoster and cytomegalovirus.14 The two most common manifestations of viral posterior uveitis are acute retinal necrosis (ARN) and progressive outer retinal necrosis (PORN). ARN is typically found in immunocompetent patients while PORN is more common in those who are immunocompromised.14,21,22
ARN begins with painful iritis and vitritis that eventually develops areas of hemorrhagic necrotizing retinitis, which presents with retinal whitening adjacent to retinal arteritis and retinal hemorrhages. Patients may also develop occlusive retinal vasculitis, optic neuritis or preretinal neovascularization.21,23 Necrosis is typically circumferential and progression is often rapid if left untreated.14
Unlike ARN, PORN does not typically present with a vitritis or occlusive vasculitis.24 There are three main stages of PORN retinitis. The early stage involves multifocal yellow-white infiltrates often involving the macula. The middle, or established phase, presents as disseminated and extensive full-thickness retinal necrosis with minimal vasculitis or hemorrhages. The late stage presents as optic atrophy and often involves rhegmatogenous retinal detachment. Both ARN and PORN can be unilateral or bilateral and lead to severe vision loss even with treatment.22
CMV retinitis is also found mainly in patients who are immunocompromised and is a common opportunistic infection in patients with acquired immunodeficiency syndrome.25 Patients are often asymptomatic but can present with photopsia, vision loss or floaters, and are rarely ever in pain. Key characteristics include retinal necrosis and vasculitis which are often accompanied by a mild vitritis. Vasculitis and hemorrhaging are the classic presentation often called “pizza-pie” or “ketchup and cottage cheese” due to the mixed white and red areas sees in the retina. Rarely, optic neuritis can also occur.14,25
Toxocariasis and Bartonella are two additional infections that can cause posterior uveitis, although it is relatively rare. Toxocariasis is caused by the parasitic roundworms Toxocara canis or Toxocara catis, found in dog or cat feces.26 It typically presents as posterior pole or peripheral granulomas which histopathologically contain roundworm fragments.26 Additional complications include chronic endophthalmitis and retinal detachment.26 Bartonella henselae or cat scratch disease, is often caused by a cat scratch or bite.27 Ocular manifestations include uveitis, vitritis, retinitis, choroiditis and optic neuritis.27
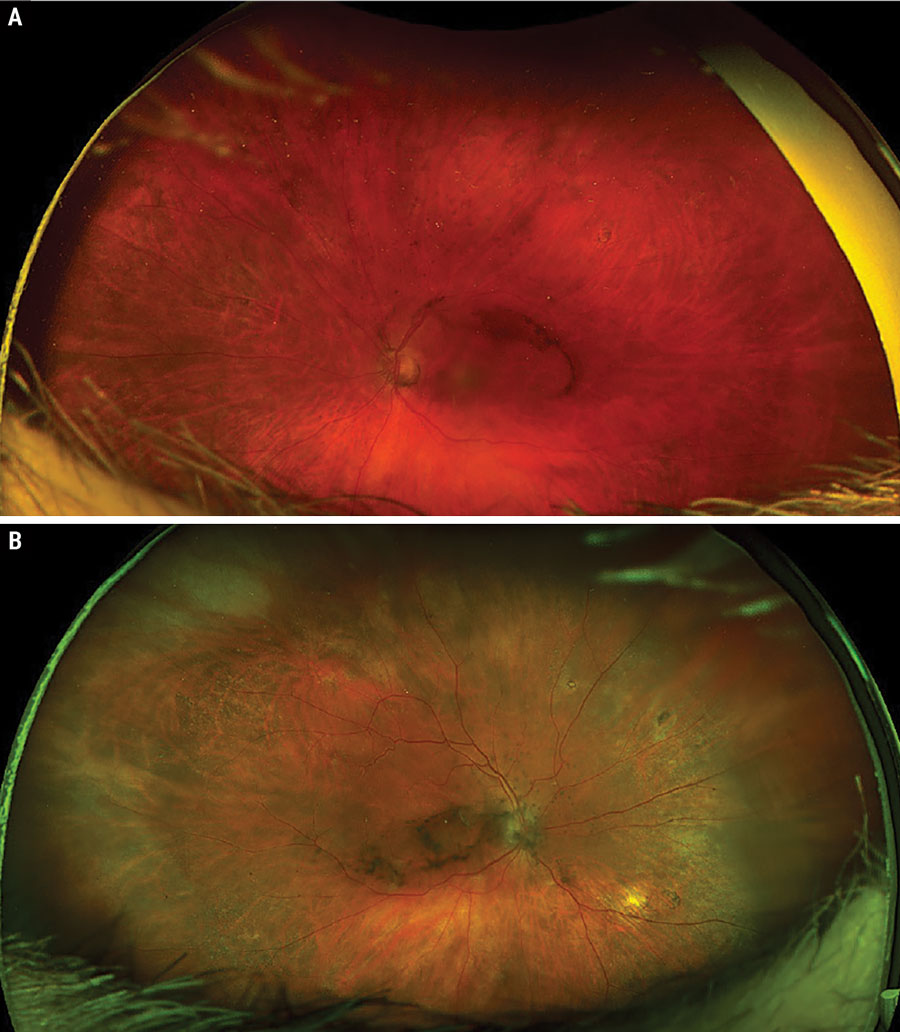 |
Bilateral posterior uveitis secondary to sarcoidosis. Click image to enlarge. |
Inflammatory
Systemic inflammatory conditions can also lead to posterior or panuveitis, although anterior uveitis is significantly more common. While any inflammatory condition can potentially cause posterior findings, we will focus on the three most likely candidates: sarcoidosis, Vogt-Koyanagi-Harada syndrome (VKH) and Beçhet’s disease.
Sarcoidosis is a multi-systemic inflammatory condition that results in granulomas developing throughout the body, most often in the lungs and lymph nodes. Ocular involvement is fairly common, with sarcoidosis being one of the most common systemic causes of uveitis. About 28% of patients with ocular sarcoidosis will develop posterior uveitis, and 9% to 30% will have panuveitis.28 Retinal periphlebitis is the most common manifestation of sarcoidosis posterior uveitis, with characteristic “candle-wax drippings” that appear as yellow or white segmented perivenous sheathing, often accompanied by vitritis.28 It is also possible to develop peripheral retinal neovascularization secondary to vascular occlusion or chronic retinal ischemia, which can present in a “sea-fan” pattern and be mistaken for sickle cell anemia, especially in African American patients.3 Multifocal choroiditis may also occur, and presents as creamy white or yellow lesions most likely in the inferior periphery.28
VKH is an idiopathic inflammatory condition that attacks melanocyte-containing tissues (including the uvea, ear and meninges) whose primary manifestation is often ocular. It is commonly associated with neurological, auditory and integumentary manifestations.29 Neurologic symptoms can include headaches and nuchal rigidity (neck stiffness); auditory symptoms may include tinnitus and/or hearing loss, and cutaneous manifestations can include vitiligo, alopecia and poliosis.
VKH usually presents as a chronic bilateral granulomatous panuveitis with exudative retinal detachments. Therefore, one of the most common complaints in patients with VKH is sudden vision loss with or without eye pain, accompanied by hearing complaints.29
There are typically three main stages of disease:
- First is the prodromal stage, occurring about one to two weeks before onset of uveitis (headache, nausea, vomiting).
- Next is the acute uveitic phase, often characterized by diffuse choroiditis, optic nerve swelling, vitritis and exudative retinal detachment.29
- Lastly, the chronic or recurrent phase involves depigmentation of the retinal pigment epithelium resulting in a “sunset glow” fundus.29 Anterior uveitis is more common in recurrent cases as well as choroidal neovascularization.
Beçhet’s disease is another chronic autoimmune disorder that can affect many different parts of the body, including the eyes, mouth, skin and genitals. It is primarily an occlusive vasculitis involving small, medium and large veins and arteries.30 Primary manifestations include uveitis (most common), oral ulcers and genital ulcers.30 Uveitis is not only the most common ocular presentation but also the most common general presentation of the disease and is often a relapsing and remitting panuveitis with retinal vasculitis.30 Initial findings may be unilateral but often progress to become bilateral. A mobile hypopyon is also observed in many cases.3 Posterior findings can be diverse, although scattered yellow or white infiltrates with surrounding hemorrhages is common and may be accompanied by vascular engorgement and/or optic disc hyperemia.30
 |
Other Etiologies
There are a few other potential etiologies for posterior and panuveitis that should be kept in mind as differentials. Primary idiopathic chorioretinopathies, or “white dot syndromes,” should be on the differential list, although they are uncommon compared to other forms of posterior uveitis.31 Multiple evanescent white dot syndrome (MEWDS) and acute posterior multifocal placoid pigment epitheliopathy (APMPPE) are two of the most common white dot syndromes.32
MEWDS usually presents in a younger population, predominantly affecting females between the age of 20 to 50 years old. Typically, MEWDS presents unilaterally with small white granular dots in the outer retina and patients often will have a viral “flu-like” prodrome of symptoms in cases of MEWDS.33 Similarly, APMPPE affects younger patients in age range of 20 to 30 years old. APMPPE does not show any gender predilection and in contrast to MEWDS, presents bilaterally with larger gray or white placoid shaped lesions within the layers of the RPE and inner choroid. Fortunately, both MEWDS and APMPPE have a good visual prognosis and are self-limiting conditions that usually only require observation.31,32
Takeaways
Posterior uveitis and panuveitis are common causes of inflammation in the eye that can cause significant and irreversible vision loss. Early recognition and detection is important to recognize the symptoms and signs early to help minimize vision loss. Because of the many different etiologies (infectious, inflammatory and ocular syndromes) that can be causing the uveitis, developing good strategies and approaches to aid in prompt diagnosis are essential to good patient outcomes. A good history and examination will help guide your differential diagnosis and medical decision-making. Ancillary testing like fundus photos, FAF, OCT and FA can help identify areas of the eye that are affected by the inflammation. Laboratory tests are also a useful tool in identifying the underlying systemic cause of the uveitis.
Dr. Trennepohl graduated from the Ohio State University College of Optometry and completed an ocular disease residency at the Chillicothe and Columbus VA. She is a staff optometrist at the Chillicothe VA Medical Center and at the Lancaster VA community-based outreach clinic. Dr. Hileman graduated from Illinois College of Optometry and completed his ocular disease residency at the Chillicothe and Columbus VA. He is the Chief of the Optometry Service at the Chillicothe VA Medical Center and is board-certified by the ABCMO. Dr. Grangaard graduated from the Ohio State University College of Optometry and completed his ocular disease residency at the Chillicothe and Columbus VA. He serves as the co-residency director at the Chillicothe VA Medical Center, is a fellow of the American Academy of Optometry and is board-certified in medical optometry. They have no financial disclosures.
1. Papaliodis G. Uveitis: etiology, manifestations and diagnosis. www.uptodate.com/contents/uveitis-etiology-clinical-manifestations-and-diagnosis?search=posterior%20uveitis&source=search_result&selectedTitle=1%7E150&usage_type=default&display_rank=1. Accessed March 29, 2024. 2. Wakefield D, Chang JH. Epidemiology of uveitis. Int Ophthalmol Clin. 2005;45(2):1-13. 3. Rathinam SR, Babu M. Algorithmic approach in the diagnosis of uveitis. Indian J Ophthalmol. 2013;61(6):255-62. 4. Lukic M, Posterior Uveitis and Panuveitis. Viewpoint. viewpoint.online/handbook/ocular-pathology-atlas/posterior-uveitis-and-panuveitis/ 5. Duplechain A, Conrady CD, Patel BC, et al. Uveitis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; Jan 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK540993/ 6. Sudharshan S, Ganesh SK, Jyotrimay B. Current approach in the diagnosis and management of posterior uveitis. Indian J Ophthalmol. 2010;58(1):29-43. 7. Meleth AD, Sen HN. Use of fundus autofluorescence in the diagnosis and management of uveitis. Int Ophthalmol Clin. 2012;52(4):45-54. 8. Agarwal A, Pichi F, Invernizzi A, et al. Stepwise approach for fundus imaging in the diagnosis and management of posterior uveitis, Surv Ophthalmol. 2023;68(3):446-80. 9. Tranos P, Karasavvidou EM, Gkorou O, Pavesio C. Optical Coherence Tomography Angiography in Uveitis. J Ophthalmic Inflamm Infect. 2019;9:21. 10. Criado E, Sanchez M, Ramirez J, et al. Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics. 2010;30(6):1567-86. 11. Andreu J, Cáceres J, Pallisa E, Martinez-Rodriguez M. Radiological manifestations of pulmonary tuberculosis. Eur J Radiol. 2004;51(2):139-49. 12. Czarnowska-Cubała M, Wiglusz MS, Cubała WJ, et al. MR findings in neurosyphilis - a literature review with a focus on a practical approach to neuroimaging. Psychiatr Danub. 2013;25 Suppl 2:S153-7. 13. Barisani-Asenbauer T, Maca SM, Mejdoubi L, et al. Uveitis - a rare disease often associated with systemic diseases and infections- a systematic review of 2619 patients. Orphanet J Rare Dis. 2012;29(7):57. 14. Mandelcorn ED. Infectious causes of posterior uveitis. Can J Ophthalmol. 2013;48(1):31-9. 15. Hughes EH, Guzowski M, Simunovic MP, et al. Syphilitic retinitis and uveitis in HIV-positive adults. Clin Exp Ophthalmol. 2010;38(9):851-6. 16. Artaechevarria Artieda J, Estébanez-Corrales N, Muñoz N, et al. Spectrum of syphilitic chorioretinitis and its evolution based on multimodal imaging. Ocul Immunol Inflamm. 2022;30(7-8):1639-50. 17. Ormaechea MS, Hassan M, Nguyen QD, Schlaen A. Acute syphilitic posterior placoid chorioretinopathy: an infectious or autoimmune disease? Am J Ophthalmol Case Rep. 2019;14:70-3. 18. Gupta A, Gupta V. Tubercular posterior uveitis. Int Ophthalmol Clin. 2005;45(2):71-88. 19. Goh EJH, Putera I, La Distia Nora R, et al. Ocular toxoplasmosis. Ocul Immunol Inflamm. 2023;31(7):1342-61. 20. Bonfioli AA, Orefice F. Toxoplasmosis. Semin Ophthalmol. 2005;20(3):129-41. 21. Bonfioli AA, Eller AW. Acute retinal necrosis. Semin Ophthalmol. 2005;20(3):155-60. 22. Lo PF, Lim R, Antonakis SN, Almeida GC. Progressive outer retinal necrosis: manifestation of human immunodeficiency virus infection. BMJ Case Rep. 2015;2015:bcr2014207344. 23. Lightman S. Acute retinal necrosis. Br J Ophthalmol. 1991;75(8):449. 24. Tseng CC, Chen SN, Hwang JF, et al. Progressive outer retinal necrosis associated with occlusive vasculitis in acquired immunodeficiency syndrome. J Formos Med Assoc. 2015;114(5):469-72. 25. Taylor GH. Cytomegalovirus. Am Fam Physician. 2003;67(3):519-24. 26. Lin P. Infectious Uveitis. Curr Ophthalmol Rep. 2015;3(3):170-83. 27. Hong H, Li T, Ying Y, et al. Cat-scratch disease manifesting as uveitis and binocular fundus nodular lesions: a case report. BMC Ophthalmol. 2023;23(1):345. 28. Jamilloux Y, Kodjikian L, Broussolle C, Sève P. Sarcoidosis and uveitis. Autoimmun Rev. 2014;13(8):840-9. 29. Fang W, Yang P. Vogt-koyanagi-harada syndrome. Curr Eye Res. 2008;33(7):517-23. 30. Ksiaa I, Abroug N, Kechida M, et al. Eye and Behçet’s disease. J Fr Ophthalmol. 2019;42(4):e133-46. 31. Cozubas R, Ungureanu E, Luminita Instrate S, et al. Similarities and differences between three different types of white dot syndrome and the therapeutic possibilities. Rom J Ophthalmol. 2018;62(3):183-7. 32. Mount GR, Kaufman EJ. White Dot Syndromes. PubMed. StatPearls Publishing, 2022. March 13, 2023. Accessed May 8, 2024. 33. Tavallali A, Yannuzzi LA. MEWDS, common cold of the retina. J Ophthalmic Vis Res. 2017;12(2):132-4. |

