Retina on the RiseExperts offer pearls for detecting and managing numerous posterior segment conditions in the June 2023 issue of Review of Optometry, our 14th annual Retina Report. Featured articles cover everything from AMD staging, referral timing, interpreting vitreous opacities and using nutrition to promote retinal health. Check out the other articles featured in this issue:
|
As optometrists, we are confronted daily with retinal pathologies that necessitate proper diagnosis and management. Delaying a referral to a retina specialist may ultimately result in negative visual sequelae for the patient, including loss of vision. It is our responsibility to not only be familiar with myriad retinal pathologies, but also understand the management guidelines that may be specific for each patient. Here, we will address a variety of retinal conditions that may present and provide clinical pearls on the proper protocol for when to refer or when not to refer to a retina specialist.
A 17-year-old female presented to the clinic with a history of blunt trauma to her right eye one day prior. She went to the ER the day of the injury, but no fractures were found on imaging. She reported photophobia, eye pain, eyelid tenderness and blurry vision in the right eye since the incident occurred. She denied flashes, floaters, diplopia or headaches. Best-corrected visual acuity (BCVA) measured 20/40 OD with no improvement with pinhole and 20/20 OS. External evaluation revealed swelling and ecchymosis of the upper and lower right eyelid. She reported pain on extraocular movements in all positions of gaze. Pupils were round and reactive without an afferent pupillary defect. There was a trace anterior chamber reaction OD. Fundus examination was unremarkable OD with a discrete area of retinal whitening superior temporal OS. The retinal vasculature was normal in appearance and the macula was flat and intact. The patient was diagnosed with commotio retinae OS and a mild traumatic uveitis OD. Refer or maintain?
Commotio retinae. This condition occurs as a result of damage to the retina due to blunt trauma to the globe. The trauma causes shock waves resulting in disruption of the photoreceptor outer segments and swelling of the retinal pigment epithelium (RPE). The retinal blood vessels in the affected area are not affected. Commotio retinae presents clinically as confluent areas of retinal whitening in the periphery or posterior pole either over the site of trauma or on the contralateral side of the globe (countercoup-180° opposite) secondary to traversing of the shock waves from the site of impact. When the macula is involved, it is referred to as Berlin’s edema, which presents clinically with a pseudo cherry red spot. Other indications of retinal trauma, such as retinal hemorrhages, may be present as well.1-3
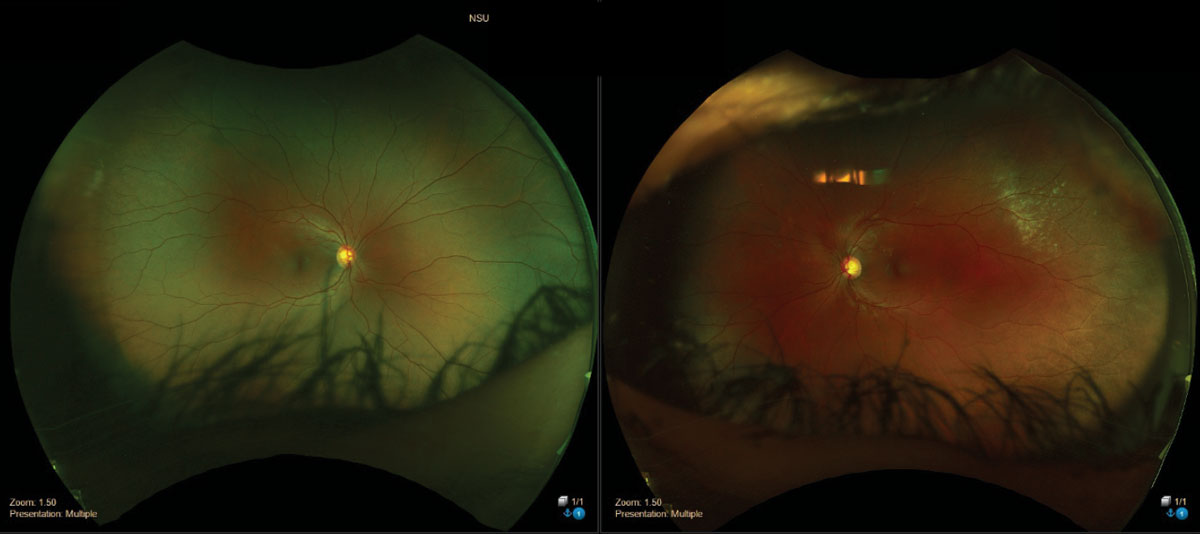 |
| Commotio retinae superior temporal of the left eye. Click image to enlarge. |
Patients will present with a history of recent ocular trauma with fogging of their vision for a few seconds to minutes after impact. The condition is painless, and symptoms are associated with the location and severity of retinal involvement. However, visual acuity does not always correlate with the degree of retinal whitening. If there is intraretinal fluid or cystoid macular edema, patients may experience decreased vision or metamorphopsia. Any patient presenting with ocular trauma needs a dilated fundus examination with or without scleral depression and ocular ultrasound (avoid scleral depression in cases of hyphema, ruptured globe or ocular inflammation).
There is no treatment for commotio retinae, with full recovery one to four weeks after presentation. Management should include addressing any comorbidities including orbital or facial fractures, corneal/conjunctival lacerations, uveitis and hyphema. Some patients may have residual RPE changes including atrophy or hyperpigmentation. In the majority of cases, vision will return to pre-injury levels, while those with macular involvement may have longstanding vision impairment.4-6 Close follow-up including monitoring of VA, amsler grid and OCT can aid in assessing the staging of the condition. Proper protocol and follow-up are essential to monitor for the development of other sequelae of the trauma.1-3
This patient was asked to return to the clinic in one week for a dilated fundus examination. She was advised to report to the clinic or ER immediately if she noted any new symptoms including changes in her vision.
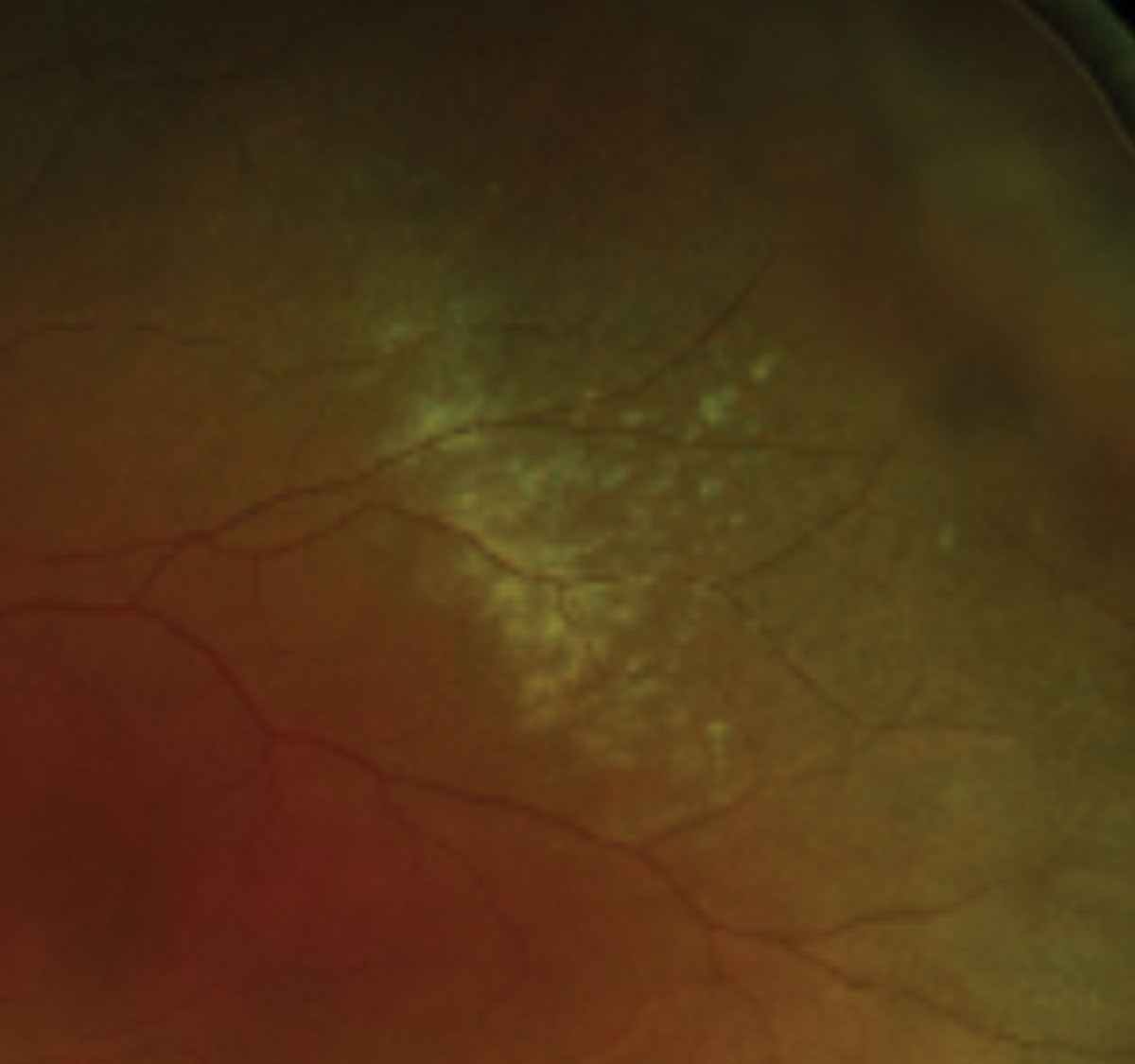 |
|
Magnified view of commotio retinae highlighting a confluent area of retinal whitening in the mid-periphery. Click image to enlarge. |
Case #2
A 49-year-old Hispanic male complained of a recent-onset large floater in his left eye that started two days prior. He also reported two episodes of light flashes that had since been resolved. The patient denied any darkening or curtains over his vision. His ocular history was positive for myopia (-5.25D OU). BCVA measured 20/20 OD and OS. Biomicroscopy was unremarkable. Inspection of the anterior vitreous revealed pigmented cells OS. Fundus examination revealed a horseshoe retinal tear inferior nasal with pronounced vitreous condensation in the peripapillary region. Refer or maintain?
Horseshoe tear. These are full-thickness retinal breaks that appear as u-shaped or flap and occur due to significant localized vitreoretinal traction. Anomalous posterior vitreous detachment (PVD) is the most common etiology of retinal horseshoe tears. PVD is an insidious process that is a normal result of aging and occurs secondary to the liquefaction or synchesis of the vitreous and anterior contraction or syneresis of the vitreous body. Flap tears occur from spontaneous PVD, where the posterior hyaloid exerts traction on the retina, pulling it anteriorly towards the vitreous cavity, resulting in a retinal tear.
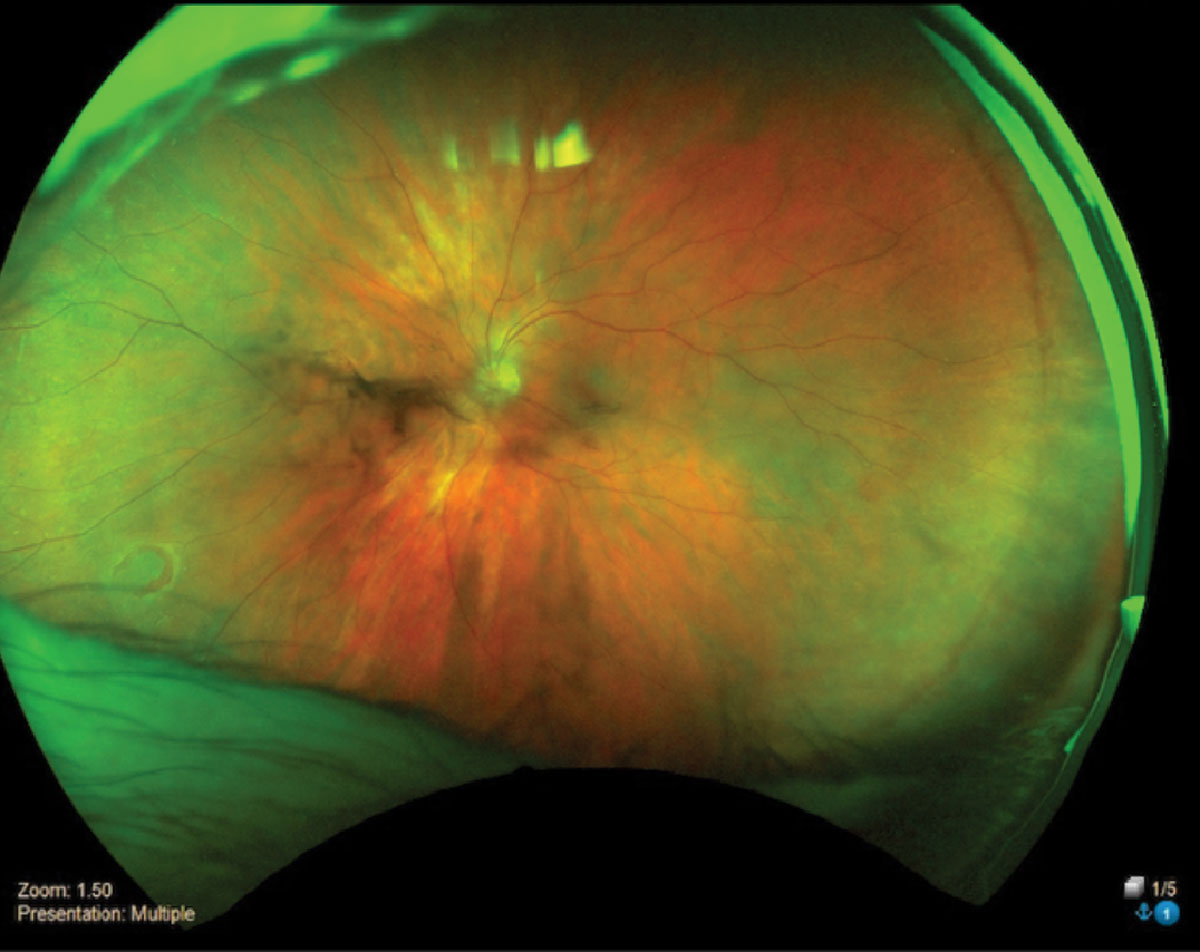 |
|
Horseshoe retinal tear inferior temporal OS. Click image to enlarge. |
Tears can exist in any region of the peripheral retina, most often near the posterior margin of the vitreous base in areas of lattice degeneration, pigment clumps or retinal tufts. With chronicity, RPE hyperplasia may develop around the lesion. This reactive pigmentation acts as a retinal “seal” and may reduce the likelihood of retinal detachment development.
Certain risk factors predispose patients to the development of a horseshoe retinal tear. These include advancing age, myopia, lattice degeneration, trauma, retinoschisis, family or personal history of retinal detachment or previous ocular surgeries (aphakia/pseudophakia). There is no prophylactic treatment for the development of horseshoe retinal tears.
Patients with acute retinal breaks may present with flashes of light and/or floaters in their vision with or without vision changes. If the retinal break is longstanding, patients may be asymptomatic. Ocular examination should include dilated fundus examination with scleral depression. Careful inspection of the anterior vitreous is critical to evaluate for the presence of pigment (Shafer’s sign), an indication of retinal break in patients without previous ocular surgeries. B-scan ultrasonography is useful if there are dense opacities obscuring accurate visualization of the retina.
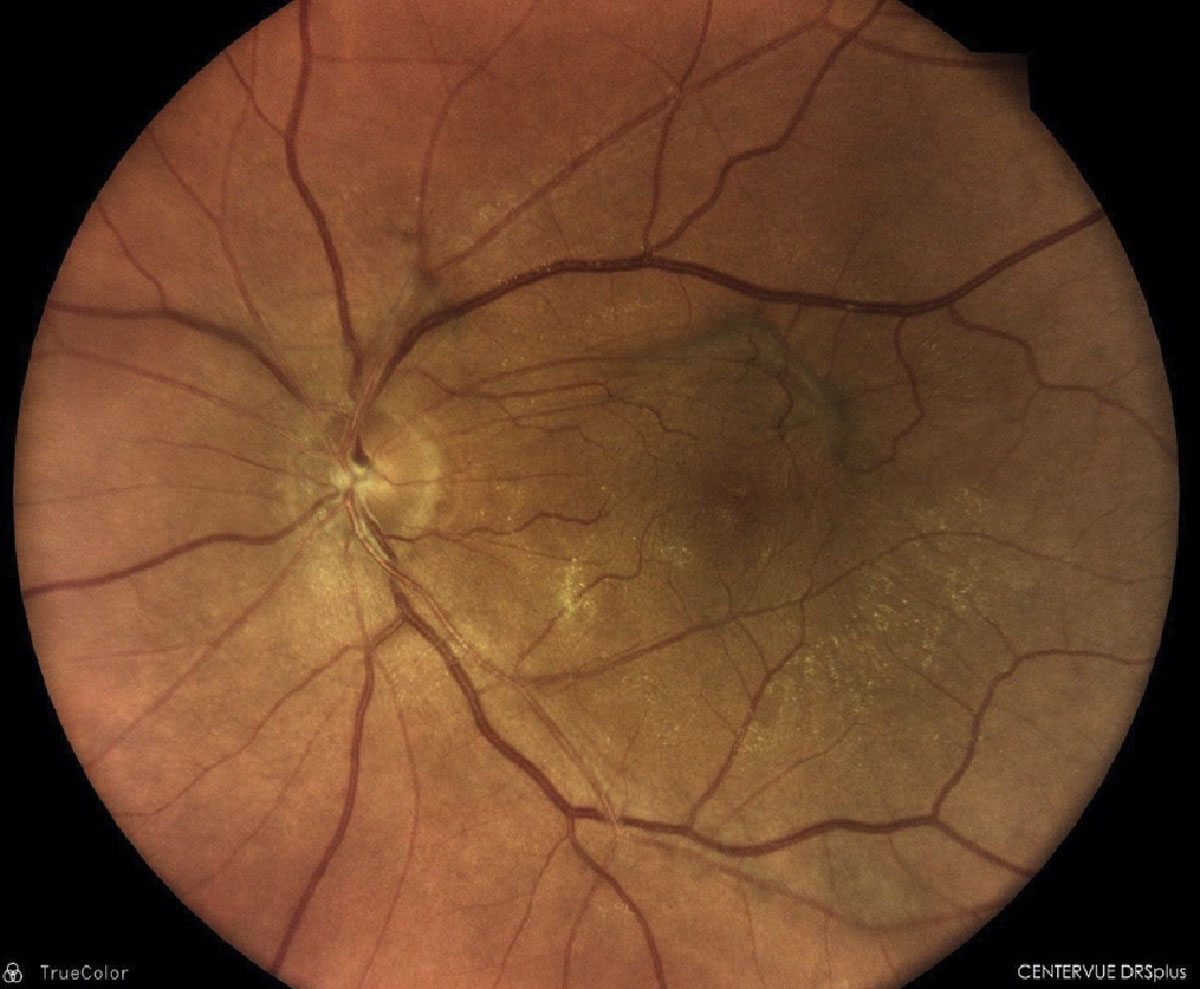 |
Epiretinal membrane with overlying vitreomacular traction membrane OS. Click image to enlarge. |
Horseshoe flap tears are the leading cause of rhegmatogenous retinal detachments, where a retinal break leads to the accumulation of subretinal fluid and separation of the neurosensory retina from the underlying RPE. This is secondary to the persistent traction of the vitreous on the apex of the flap. Symptomatic horseshoe tears have a 33% to 55% chance of progressing to retinal detachment. Asymptomatic flap tears with subretinal fluid have a 40% chance of progressing while those without subretinal fluid have a 5% to 10% chance of progressing.7-12
Acute symptomatic retinal breaks require immediate laser therapy or cryotherapy (freezing procedure) within 24 hours. The goal of treatment is to provide a chorioretinal seal around the lesion preventing vitreous from getting into and under the break resulting in a detachment. Treatment for asymptomatic horseshoe tears is case dependent. Observation without treatment may be appropriate for chronic tears, tears surrounded by pigmentation, tears without vitreoretinal traction or other asymptomatic patients. Following prophylactic laser treatment, the patient should be seen at one week and again at three to six weeks. Patients with risk factors or those with breaks that do not require treatment should be followed at three months and then every six to 12 months if stable.7-12
This patient was immediately referred to the retina specialist for intervention. Horseshoe tears should be sent to the retina specialist the same day or at the latest the following morning.
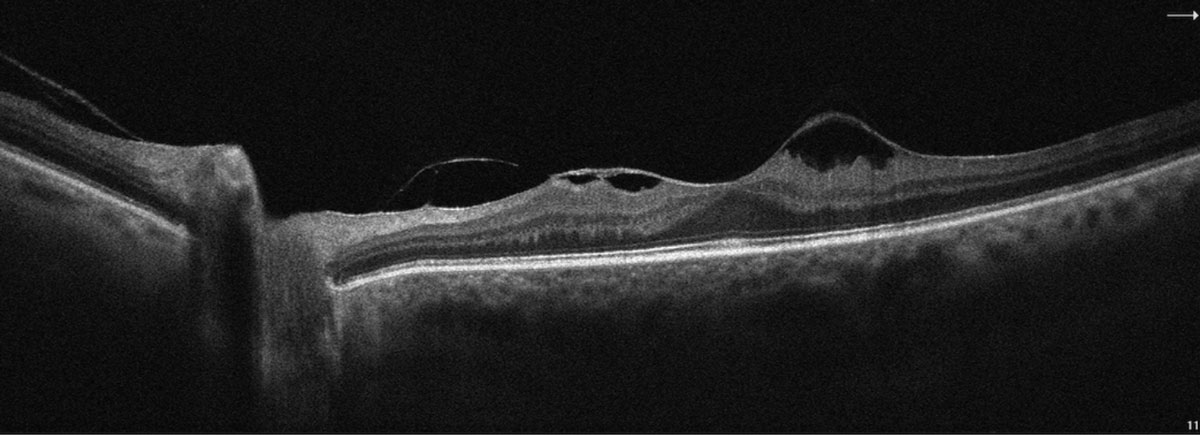 |
|
SD-OCT image highlighting vitreomacular traction with epiretinal membrane OS. Click image to enlarge. |
Case #3
A 60-year-old male presented with complaints of blurry vision and metamorphopsia OU. He stated that he started noticing the disturbance the previous year, but it got progressively worse. His ocular history was positive for successful cataract extraction two years prior. BCVA was 20/30 OD and 20/40 OS. His entrance testing was unremarkable; however, he did note squiggling of the lines on Amsler grid OS and a smudge in the center of his vision OD. Fundus examination revealed a maroon, well-defined circular lesion directly over the fovea OD and an epiretinal membrane with visible vitreous traction suspending from the optic nerve hypoplasia superior to the macula OS. Watzke Allen test was negative OD and OS. OCT confirmed the presence of a partial thickness hole (lamellar) OD and epiretinal membrane (ERM) with vitreomacular traction (VMT) OS. Refer or maintain?
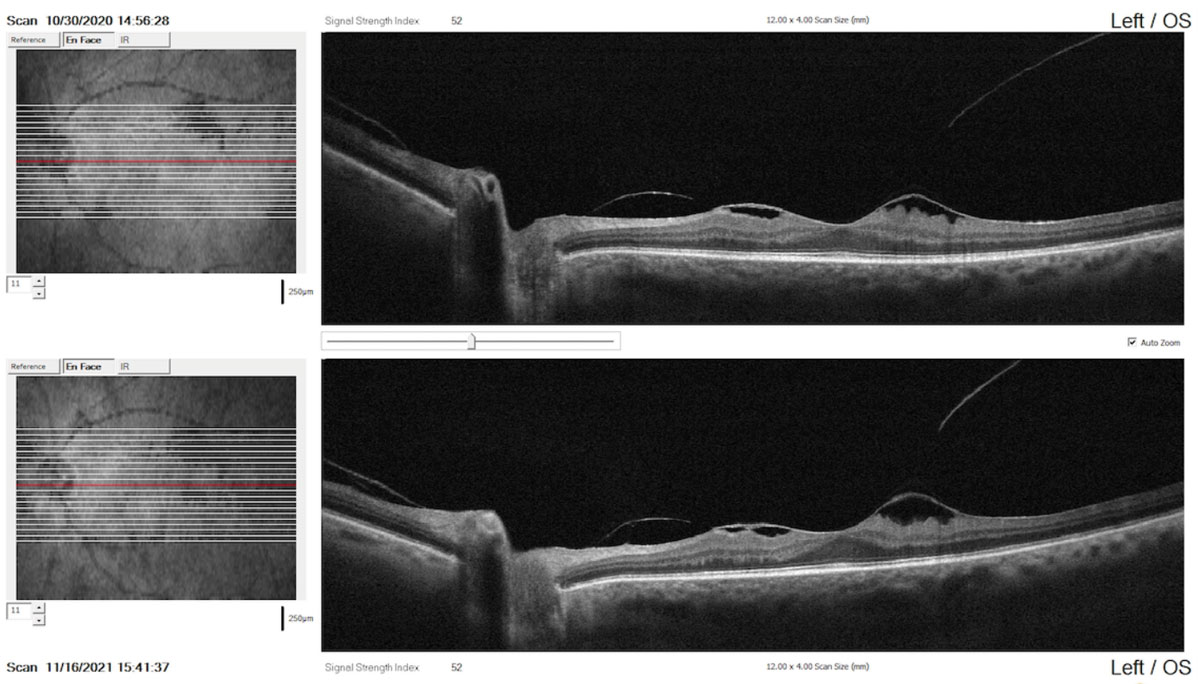 |
Comparison report with raster scans performed one year apart indicating the stability of the condition (top: baseline scan; bottom: recent scan). Click image to enlarge. |
ERM. This growth is defined as a fibrocellular proliferation of the superficial retina. The proliferation forms a scaffold that is composed of cellular contents, vitreous and fibrotic components and is associated with glial cell proliferation. A fine-to-thick glistening membrane will be seen on clinical examination. The incidence of ERM varies widely in the literature, from 2.2% to 11% in phakic patients without pre-existing ocular conditions.13,14 Studies have shown that 2% of patients over the age of 50 and 20% over the age of 75 have evidence of ERMs, although most do not need treatment. Both sexes are equally affected. In about 10% to 20% of cases, both eyes have ERMs, but they can be of varying degrees of severity.15 The most common etiology of ERM is an anomalous PVD with trauma, retinal breaks, retinal vascular diseases, retinal inflammation as well as others.
 |
|
Full-thickness macular hole with surrounding fluid cuff. Click image to enlarge. |
The pathophysiology behind ERM formation involves the presence of retinal microbreaks in the inner limiting membrane (ILM) allowing for microglial cells to gravitate and diffuse onto the retinal surface. The migration of these cells results in firm adherence of the resulting membrane to the underlying retina.16-18 ERM can also lead to macular traction and symptomatology. If the residual vitreous exerts traction on the center 3mm radius of the fovea, resulting in a foveal contour change, VMT results. ERM and VMT can lead to vision loss, metamorphopsia and doubling of images.
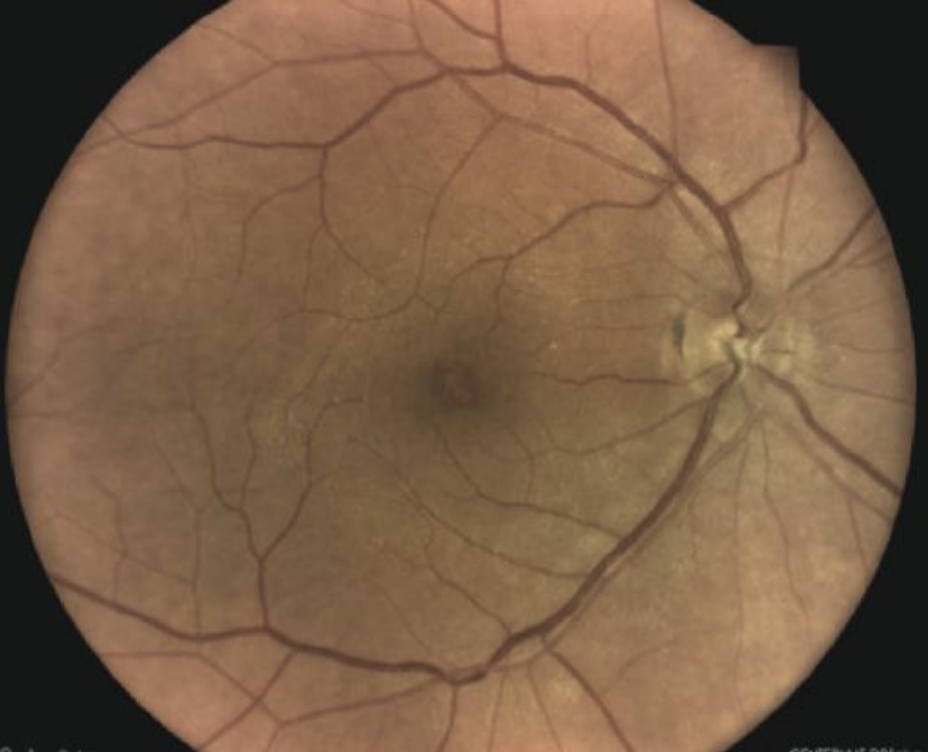 |
| Macular pseudohole. Click image to enlarge. |
OCT is a highly effective way of imaging both ERM and VMT and is used to make management/treatment decisions in affected patients. Three-dimensional analysis of the retinal architecture from inner limiting membrane through choroid allows for visualization of not only superficial retinal changes but also inspection of the photoreceptor integrity line and outer retina to assess for the extent of involvement.
Epiretinal membranes tend to remain stable and can be monitored when the patient is asymptomatic. However, if a patient’s vision is 20/60 or worse, if they are symptomatic (metamorphopsia or doubling of vision) or if there is evidence of progression, they should be referred for possible surgical intervention. The most common surgery for ERM is pars plana vitrectomy with membrane peel with or without concomitant peeling of the ILM. Visual recovery can be slow ranging from three to 12 months postoperatively. Recurrence of membranes occurs in approximately 5% of patients.19
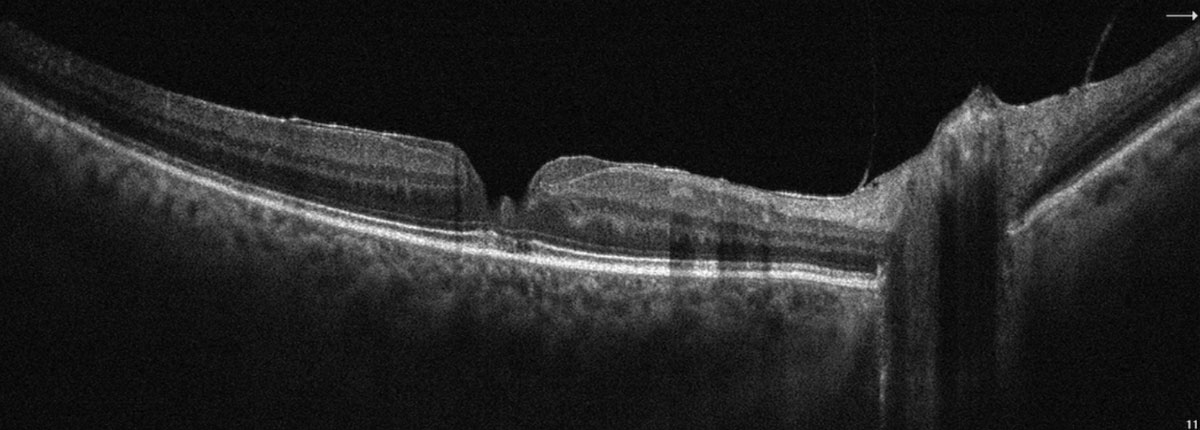 |
|
SD-OCT highlighting a partial thickness macular pseudohole with overlying epiretinal membrane. Click image to enlarge. |
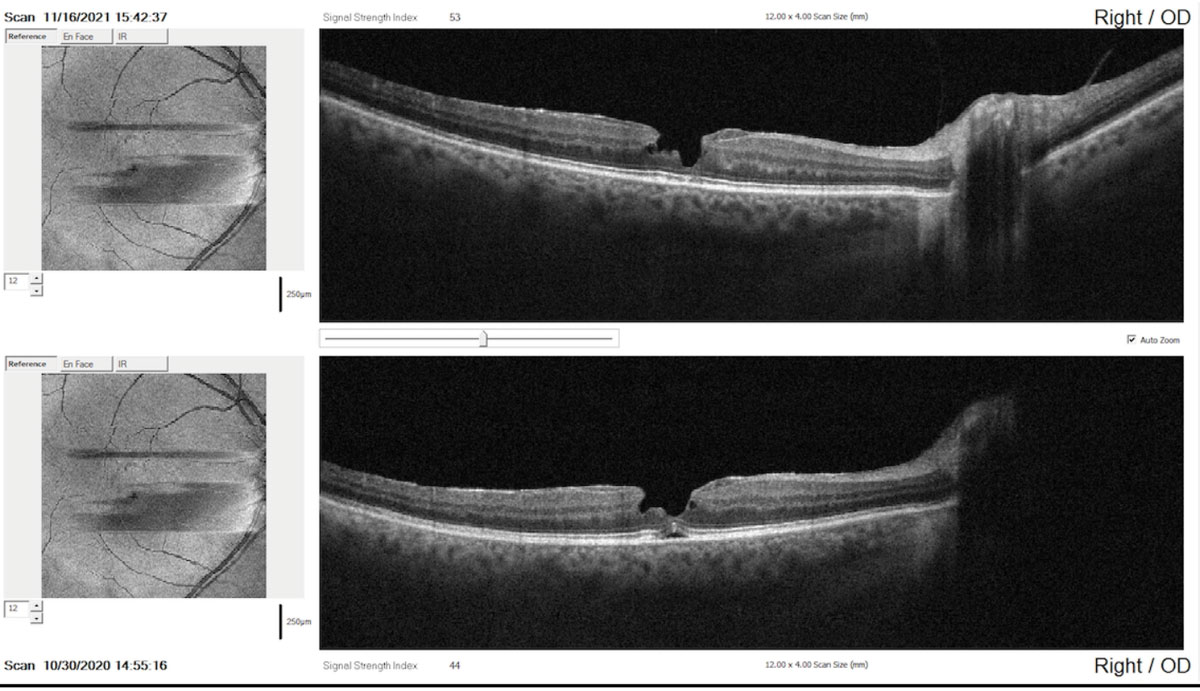 |
|
SD-OCT comparison report taken one year apart showing minor evidence of change in the contour and irregularity of the pseudohole (top: recent scan; bottom: baseline scan). Click image to enlarge. |
VMT. In these cases, if the patient is asymptomatic, regular monitoring with OCT (three-month intervals) and daily home amsler grid use is recommended. VMT spontaneously resolves in 11% to 53% of cases with mean time of release of 15 to 18 months. This statistic highlights the variability in the course of the condition.20-24 If the patient becomes symptomatic, they should be referred to a retinal specialist, as VMT can lead to macular hole, ERM or cystoid macular edema. Pars plana vitrectomy is one treatment option that involves the manual release of the vitreous attachment thereby alleviating the traction. Pars plana vitrectomy can work well in cases of pseudophakia, concurrent ERM and broad vitreomacular adhesions.25-27 Pneumatic vitreolysis is a procedure that involves injecting a small gas bubble into the eye which severs the adhesion between the vitreous and the macula inducing a PVD.28,29 Due to the increased rate of retinal tears and detachments, this procedure is no longer being used.30
A partial thickness macular hole was found in the other eye. How do we differentiate it from a full-thickness macular hole?
Full-thickness macular hole is a localized, complete loss of tissue affecting the ILM layer through the RPE. During clinical exam, it will appear as a circular, reddish lesion over the fovea. The edge of the hole may contain diffuse areas of intraretinal pseudocysts that will appear as circular hyporeflective lesions.
Due to the wide variability in defining macular holes, The International Vitreomacular Traction Study Group provided a universal classification system using findings seen on OCT. The classification system evaluated two aspects of the hole. First, they evaluated the size of the hole using OCT metrics. If the hole measured <250µm at the narrowest point of separation, it was considered small. If the hole was 250µm to 400µm it was considered medium, and >400 µm was considered large. These measurements provide a means of determining efficacy and outcome of treatment. The second parameter that was assessed was the relationship of the vitreous to the hole. If there was vitreomacular traction or vitreous involvement, this would be considered a primary full-thickness hole and if the vitreous was not involved, then it would be considered a secondary hole. Examples of causative factors resulting in a secondary macular hole are trauma, high myopia and macular schisis.31 Most full-thickness macular holes should be referred to a retina specialist; however, longstanding holes will not improve from intervention and thus may be monitored.
Lamellar macular hole is a partial-thickness macular hole with an incomplete loss of tissue from ILM to RPE. During clinical exam, it will appear as a circular or oval reddish lesion similar to a full-thickness macular hole. On OCT, lamellar holes may have: an irregular foveal contour, a defect in the inner fovea which does not have to be actual loss of retinal layers or schisis between the outer plexiform layer and outer nuclear layer in the presence of an intact photoreceptor layer.
Macular pseudoholes are also partial-thickness holes that resemble a “U” shape and can be related to ERM. When should we refer partial-thickness holes? Surgical intervention tends to be controversial as results are variable. There may be reason to recommend surgery in patients with partial-thickness macular holes; particularly those with functional or anatomical deterioration, those experiencing metamorphopsia or those who have poor entering visual acuity resulting in inability to perform daily tasks. Other guidelines recommend surgical intervention whenever VA <20/40 and there is evidence of ERM.32,33 Surgical options include phacoemulsification with vitrectomy and ERM and ILM peeling. Preoperative defects in the photoreceptor integrity line are associated with worse visual prognosis.34
Due to complaints of metamorphopsia and blurry vision, we opted to refer this patient to a retina specialist for consultation. Surgical intervention was not performed; however, the patient is being comanaged by our clinic and the retina specialist.
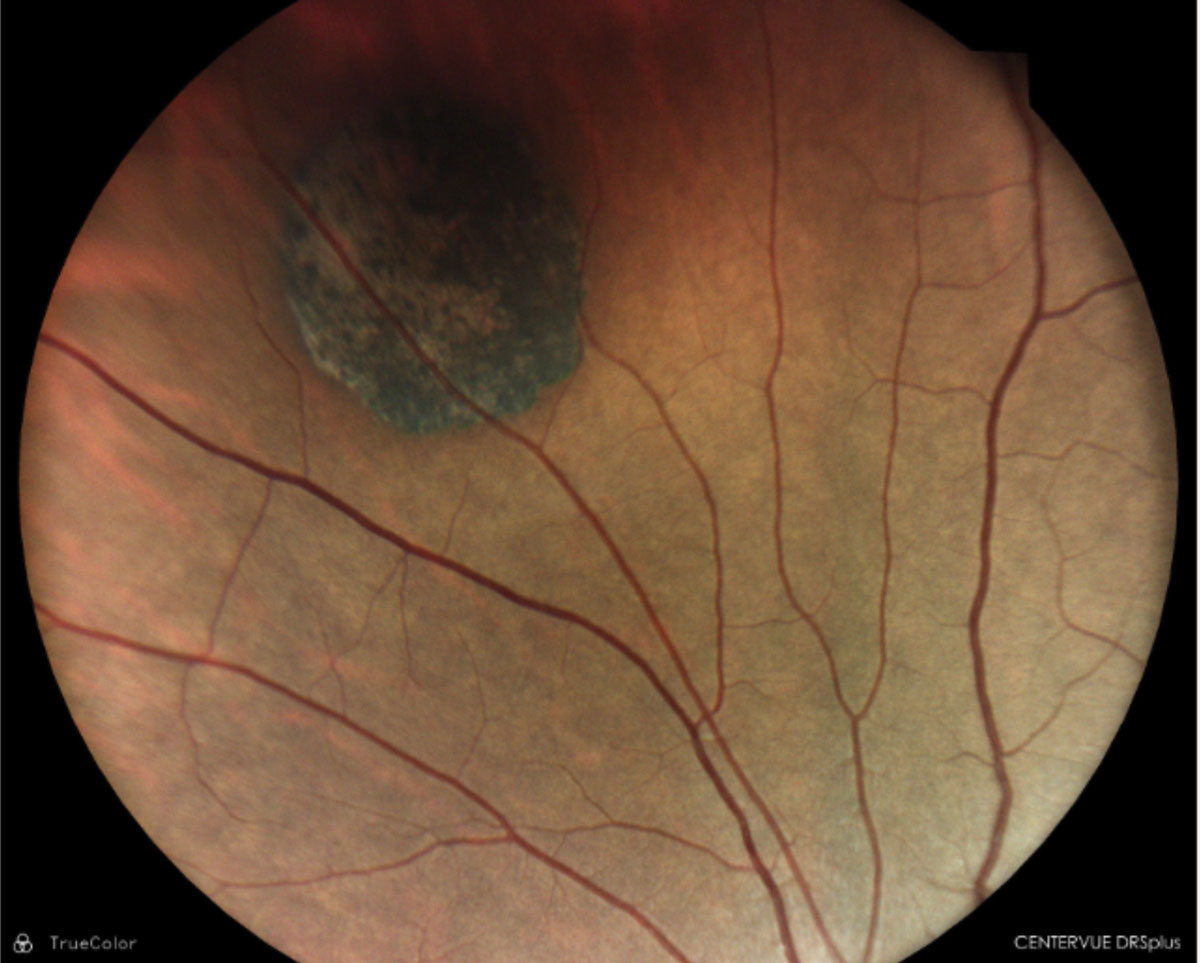 |
|
CHRPE; well-demarcated lesion with lacunae within. Click image to enlarge. |
Case #4
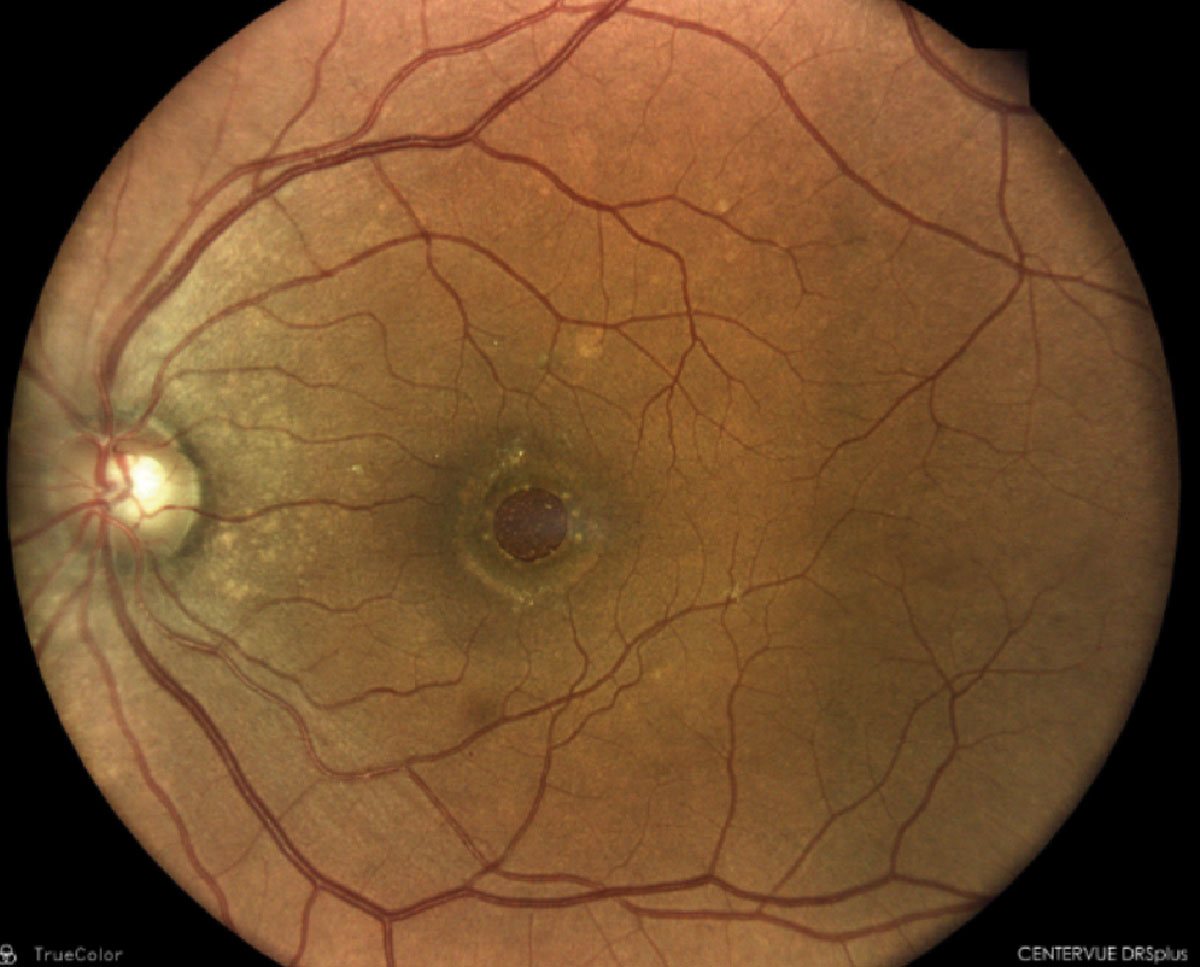
A 45-year-old Hispanic female presented for a comprehensive eye examination. She reported mild blurry vision without her glasses but had no other visual complaints. Her ocular and medical history were unremarkable. Her family medical history was positive for skin cancer in her father and uterine cancer in her mother; both parents were still alive. Entrance testing including slit lamp biomicroscopy were unremarkable with BCVA 20/20 OD and OS. Funduscopy revealed a solitary, mid-peripheral 6-7DD darkly pigmented, well-defined lesion with lacunae within. The remainder of the fundus examination was unremarkable OU. Ancillary testing including OCT was obtained on the day of the visit. Refer or maintain?
Congenital hypertrophy of the retinal pigment epithelium (CHRPE). These are benign pigmented hamartomas of the retina that are routinely found on comprehensive eye examination. These lesions present clinically as well-defined, round to oval gray, brown to black lesions, while atypical amelanotic variants also exist. CHRPE are histologically composed of enlarged, taller RPE cells that are tightly packed with melanin granules. Defining characteristics of these lesions include hypopigmented borders or halo around the lesion with lacunae or pale colored areas within. Such lesions may present as unifocal or multifocal and may be present in the mid-peripheral or peripheral fundus.
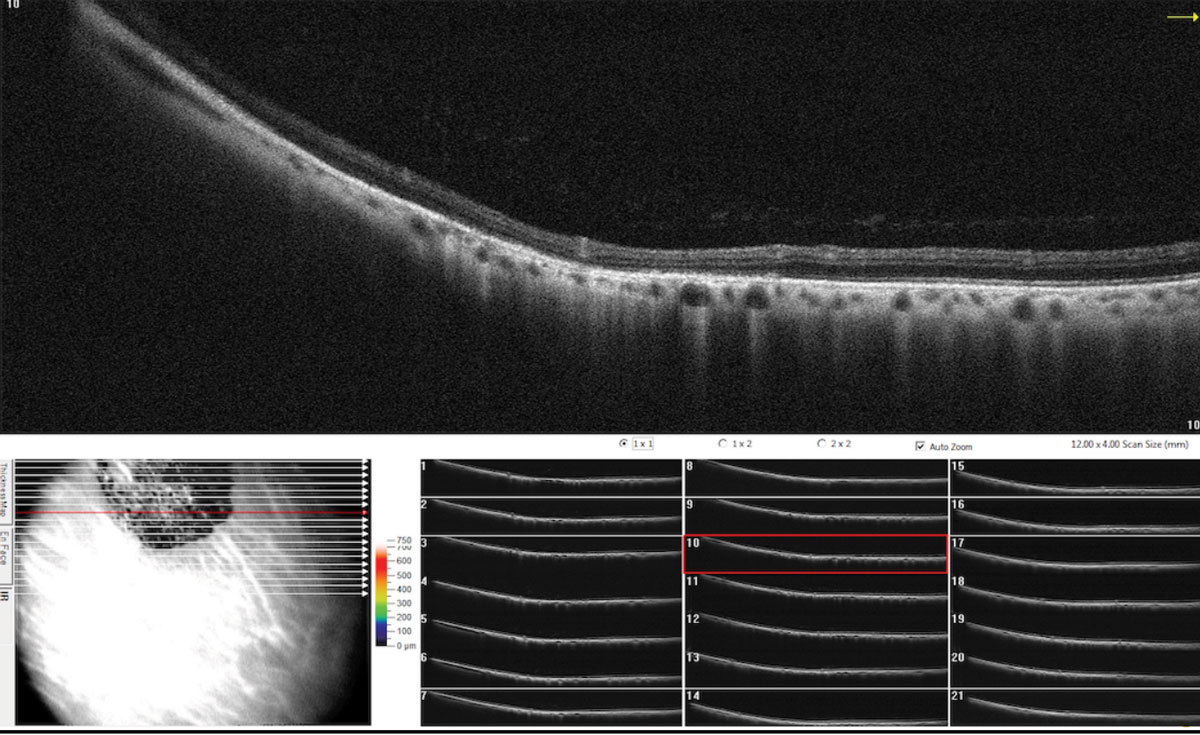 |
|
SD-OCT raster scan through the CHRPE illustrating retinal thinning above the lesions with photoreceptor loss and shadowing of underlying choroid. Click image to enlarge. |
Grouped CHRPE lesions are often referred to as “bear tracks.” Atypical CHRPE is associated with familial adenomatous polyposis, including subtypes Gardner’s syndrome, familial polyposis coli and Turcot syndrome; these are malignant conditions characterized by polyps of the colon and rectum.
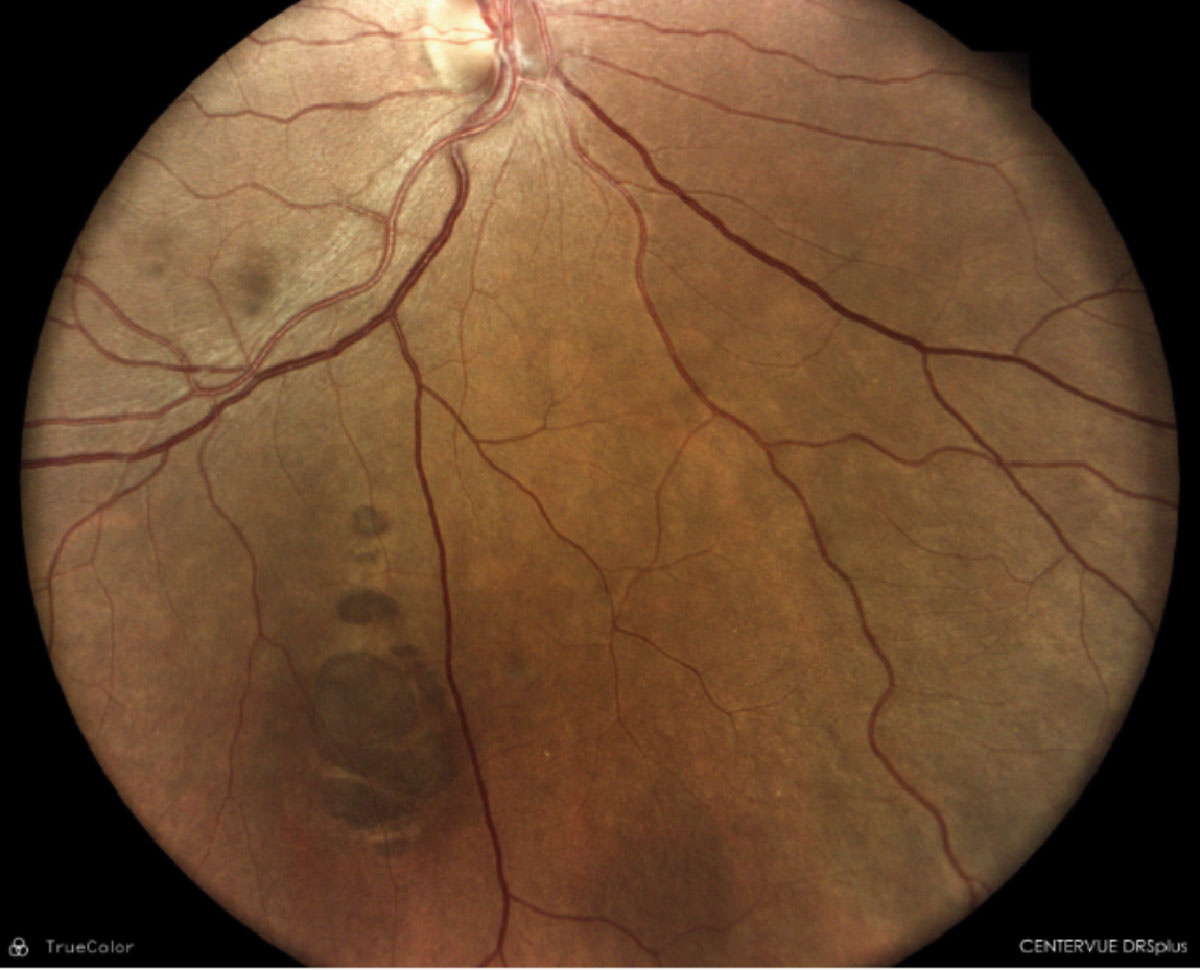 |
|
Scattered CHRPE with depigmented tails and irregular borders. Click image to enlarge. |
The presence of multiple scattered, bilateral CHRPE with depigmented tails and irregular borders are considered a clinical disease marker and can be used to identify at-risk patients.35 It has been proposed that four or more of these lesions is highly suggestive for familial adenomatous polyposis.36-38
Diagnostic imaging modalities include fluorescein angiography and indocyanine green angiography; both of which will show blockage of fluorescence of the pigmented lesions with transmission of choroidal fluorescence through the depigmented areas throughout the angiogram. Fundus autofluorescence can rule out lesions that simulate melanoma. Visual field findings of CHRPE are variable and include relative to absolute scotomas depending on the extent of the lesion. OCT will exhibit retinal thinning above the lesion with photoreceptor loss and shadowing of the underlying choroid.39,40 Ultrasonography can be used to document thickness and confirm clinical findings; ultrasound of a melanotic lesion usually shows low-to-moderate reflectivity with choroidal excavation.
Management includes periodic observations. If evidence of subretinal fluid or exudation appears, or if the lesion has characteristics suspicious for malignancy, they should be referred to a retinal specialist for intervention. Lesions that are atypical in appearance should warrant colorectal screening with a gastroenterologist.
Back to our patient, the clinical appearance and ancillary testing was highly suggestive of typical unifocal CHRPE and thus we opted to monitor the patient annually. The patient was advised regarding the necessity and importance of regular dilated eye examinations.
Takeaways
As primary eyecare doctors, optometrists are exposed to an endless array of ocular conditions. It is our responsibility to be familiar with the proper diagnosis and management of these conditions, as timely referral may make a big difference in the final outcome. We are often the first point of contact for our patients, and with this comes an obligation to know when to hold and when to refer to our patient.
Dr. Rodman is a professor and chief of the Fort Lauderdale (Broward) Eye Care Institute at Nova Southeastern University in Florida. She is a consultant and speaker for Visionix (Optovue), iCare, LKC Technologies, Iveric Bio, Apellis and OcuTerra. Dr. Herring graduated from Nova Southeastern Univeristy College of Optometry (NSUCO) last year and is currently completing a residency in primary care with an emphasis in pediatric and binocular vision at NSUCO. She has no financial interests to disclose.
1. Mansour AM, Green WR, Hogge C. Histopathology of commotio retinae. Retina. 1992;12(1):24-8. 2. Gervasio K, Peck T. The Wills Eye manual: office and emergency room diagnosis and treatment of eye disease. Lippincott Williams & Wilkins, 2021. 3. Sipperley JO, Quigley HA, Gass DM. Traumatic retinopathy in primates. The explanation of commotio retinae. Arch Ophthalmol. 1978;96(12):2267-73. 4. McGwin G Jr, Owsley C. Ocular morbidity associated with airbag deployment: a report of seven cases and a review of the literature. Arch Ophthalmol. 2005;23(5):662-6. 5. Park JY, Nam WH, Kim SH, et al. Evaluation of the central macula in commotio retinae not associated with other types of traumatic retinopathy. Korean J Ophthalmol. 2011;25(4):262-7. 6. Chen AJ, Linakis JG, Mello MJ, Greenberg PB. Epidemiology of infant ocular and periocular injuries from consumer products in the United States. 2001-2008. J AAPOS. 2013;17(3):239-42. 7. Davis MD. Natural history of retinal breaks without detachment. Arch Ophthalmol 1974;92(3):183-94. 8. Goldberg RE, Boyer DS. Sequential retinal breaks following a spontaneous initial retinal break. Ophthalmol. 1981;88:10-2. 9. Preferred Practice Patterns: Posterior vitreous detachment, retinal breaks and lattice degeneration. AAO 2019. 10. American Academy of Optometry. Care of the patient with retinal detachment and related peripheral vitreoretinal disease. 1995. 11. Kanski JJ, Bowling B. Clinical Ophthalmology, a Systematic approach, 7th edition. Butterworth-Heinemann Elsevier; 2011. 12. Yanoff M, Duker JS Ophthalmology, 3rd edition, Chapter 6.73 - Retinal Breaks, Craig M. Geven. 13. Klein BR, Brown EN, Casden RS. Preoperative macular spectral-domain optical coherence tomography in patients considering advanced-technology intraocular lenses for cataract surgery. J Cataract Refract Surg. 2016;42(4):537-41. 14. Zafar S, Siddiqui MAR, Shahzad R, Shahzad MH. Swept-source optical coherence tomography to screen for macular pathology in eyes having routine cataract surgery. J Cataract Refract Surg. 2017;43(3):324-7. 15. Savingvision.org 16. Foos RY. Vitreoretinal juncture; epiretinal membranes and vitreous. Invest Oph Vis Sci. 1977;16(5):416-22. 17. Wolter JR. Pores in the internal limiting membrane of the human retina. Acta Ophthalmol. 1964;42:971-4. 18. Sebag J. Anomalous posterior vitreous detachment: A unifying concept in vitreo-retinal disease. Graefes Arch Clin Exp Ophthalmol. 2004;242(8):690-8. 19. Hartmann KI, Schuster AK, Bartsch D-U, et al. Restoration of retinal layers after epiretinal membrane peeling. Retina. 2014;34(4):647-54. 20. Hikichi T, Yoshida A, Trempe CL. Course of vitreomacular traction syndrome. Am J Ophthalmol. 1995;119(1):55-61. 21. Odrobina D, Michalewska Z, Michalewski J, et al. Long-term evaluation of vitreomacular traction disorder in spectral-domain optical coherence tomography. Retina. 2011;31:324-31. 22. John VJ, Flynn HW, Jr., Smiddy WE, et al. Clinical course of vitreomacular adhesion managed by initial observation. Retina. 2014;34(3):442-6. 23. Charalampidou S, Nolan J, Beatty S. The natural history of tractional cystoid macular edema. Retina. 2012;32(10):2045-51. 24. Tzu JH, John VJ, Flynn HW, Jr., et al. Clinical course of vitreomacular traction managed initially by observation. Ophthalmic Surg Lasers Imaging Retina. 2015;46(5):571-6. 25. Smiddy WE, Michels RG, Glaser BM, deBustros S. Vitrectomy for macular traction caused by incomplete vitreous separation. Arch Ophthalmol. 1988;106(5):624-8. 26. Davis RP, Smiddy WE, Flynn HW, Jr., et al. Surgical management of vitreofoveal traction syndrome: optical coherence tomographic evaluation and clinical outcomes. Ophthalmic Surg Lasers Imaging. 2010;41(2):150-6. 27. Jackson TL, Nicod E, Angelis A, et al. Pars plana vitrectomy for vitreomacular traction syndrome: a systematic review and meta-analysis of safety and efficacy. Retina. 2013;33(10):2012-7. 28. Sayegh RG, Georgopoulos M, Geitzenauer W, et al. High-resolution optical coherence tomography after surgery for vitreomacular traction: a two-year follow-up. Ophthalmology. 2010;117(10):2010-7.e1-2. 29. Stalmans P, Girach A. Vitreous levels of active ocriplasmin following intravitreal injection: results of an ascending exposure trial. Invest Ophthalmol Vis Sci. 2013;54(10):6620-7. 30. Chan CK, Mein CE, Glassman AR, et al. Pneumatic vitreolysis with perfluoropropane for vitreomacular traction with and without macular hole: DRCR Retina Network Protocols AG and AH. Ophthalmology. 2021;128(11):1592-603. 31. Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611-9. 32. Sun, JP, Chen SN, Chuang CC, et al. Surgical treatment of lamellar macular hole secondary to epiretinal membrane. Graefes Arch Clin Exp Ophthalmol. 2013;251(12):2681-8. 33. Lai TT, Chen SN, Yang CM. Epiretinal proliferation in lamellar macular holes and full-thickness macular holes: clinical and surgical findings. Graefes Arch Clin Exp Ophthalmol. 2016;254(4):629-38. 34. Figueroa MS, Govetto A, Steel DH, et al. Pars plana vitrectomy for the treatment of tractional and degenerative lamellar macular holes: functional and anatomical results. Retina. 2019;39(11):2090-8. 35. Laghmari M, Lezrek O. Congenital hypertrophy of the retinal pigment epithelium in Gardner’s syndrome. Pan Afr Med J. 2014;19:164. 36. Shields JA, Shields CL. Tumors and related lesions of the pigment epithelium. In: Shields JA, Shields CL. Intraocular Tumors: An Atlas and Textbook. 3rd ed. Philadelphia: Wolters Kluwer; 2015:453-502. 37. Shields JA, Shields CL, Mercado G, et al. Adenoma of the iris pigment epithelium. A report of 20 cases: the 1998 Pan-American Lecture. Arch Ophthalmol. 1999;117:736-41. 38. Shields JA, Sanborn GE, Augsburger JJ. The differential diagnosis of malignant melanoma of the iris. Ophthalmology. 1983;90:716-20. 39. Shields CL, Materin MA, Shields JA. Review of optical coherence tomography for intraocular tumors. Curr Opin Ophthalmol. 2005;16:141-54. 40. Shields CL, Materin MA, Walker C, et al. Photoreceptor loss overlying congenital hypertrophy of the retinal pigment epithelium by optical coherence tomography. Ophthalmology. 2006;113:661-5. |