Keratoconus can be classified by the degree of conicity as early or advanced, or it can be classified morphologically by the shape of the cone.
Early keratoconus usually manifests as a small island of irregular astigmatism in the inferior paracentral cornea.
Advanced keratoconus has been classically subdivided into three categories:
Nipple. The nipple form of keratoconus is comprised of a small, near-central ectasia of 5mm or less in diameter. It is called the nipple form because there is sometimes, but not always, an elevated fibroplastic nodule at the apex of the cone.
Oval. The most common shape in advanced keratoconus is oval. This is characterized by displacement of the corneal apex below the midline, resulting in an island of inferior midperipheral steepening.
|
|
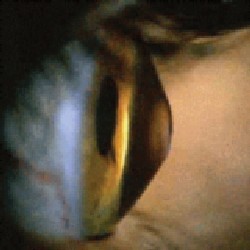
| Side profile of a cornea in a patient with keratoconus. |
Globus. The globus form of keratoconus encompasses nearly three-quarters of the corneal surface and usually has no surrounding island of normal midperipheral cornea.1
In recent years, some practitioners have abandoned the nipple- oval-globus classification due to the widespread use of corneal topographers.
Signs and Symptoms
Patients with keratoconus often complain of decreased vision, photophobia, monocular diplopia, visual distortion, asthenopia, and glare around lights. You should suspect keratoconus in any young adult with irregular astigmatism or any patient with myopic astigmatism whose eyeglass prescription is changing more frequently than normal. Your suspicions should be heightened if such a patient is not correctable to 20/20 in the phoropter.
Slit lamp findings on a patient with keratoconus include Fleischers ring, Vogts striae, corneal thinning, and Munsons sign. Also seen are increased visibility of corneal nerves, apical thinning, anterior stromal clearing lines, subepithelial fibrillary lines, and central or eccentric corneal protrusion. Fleischers ring is a yellow-brown line that demarcates the peripheral edge of the cone. Vogts striae are vertical stress lines near the apex of the cone, appearing as a series of sharp, whitish, vertical or oblique lines just anterior to Descemets membrane. In some patients, they may be horizontal as well. Slit lamp examination will also demonstrate displacement of the apex of the cornea below a hypothetical line that bisects the pupillary axis. The other common sign is Munsons sign, which is a bulging of the lower lid during downgaze.
During retinoscopy, the red reflex in a keratoconic eye often demonstrates high amounts of irregular astigmatism with a scissors motion. After dilation,
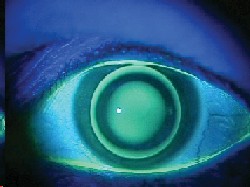
keratoconus patients sometimes demonstrate a dark annular shadow that surrounds the bright reflex at the apex of the cone. This shadow is caused by total internal reflection of light brought about by the conical-shaped cornea. In some patients, this can be seen without dilation, and it is almost always diagnostic of keratoconus.
A patient with keratoconus will exhibit several features on keratometry. In many cases, corneal astigmatism is so pronounced that the mires may appear oval. They are usually distorted to some degree by the irregular corneal surface. The central keratometric rings are frequently non-superimposable, suggesting irregular corneal astigmatisma hallmark of keratoconus.2
On corneal topography, early keratoconus reveals a characteristic pear-shaped elongation of the central mires at the midperiphery, below the corneal midline. As the condition progresses, the steepening usually spreads nasally to include the inferonasal cornea. Advanced keratoconus can demonstrate rotational steepening above the midline along a superotemporal path. The last area to be affected by keratoconus is usually the superior nasal quadrant of the cornea.1 However, there is some variability in this presentation. Some practitioners may find that the apex of the cone is decentered below the midline and temporal.
Treatment Options
Treatment of keratoconus depends on the severity of the condition. Early in the disease, eyeglasses are successful in restoring vision. However, as the disease progresses, the patient will need contact lenses for optimal visual acuity. Lens options include soft (hydrogel) lenses, basic gas permeable lenses and specialty gas permeable lenses.
| Case Study: It"s All in The Fit A 38-year-old Asian male was referred for evaluation of his keratoconus. His medical and ocular history were unremarkable except for a history of eyeglasses since age 5 and contact lens wear since age 19. Initially, he wore soft yearly contact lenses but, at the time of my exam, was wearing quarterly replacement lenses of an unknown brand. His chief complaint was poor visual acuity from both glasses and contact lenses. |
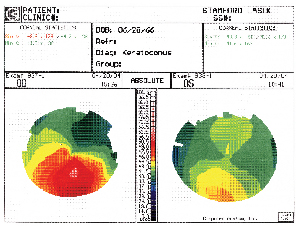 |
| His manifest refraction yielded 8.00 +6.00 x 125 O.D. with VA of 20/100 and 7.75 +3.00 x 75 O.S. with VA of 20/40. Corneal topography revealed the results shown above. I discussed with him in detail the noticeable improvement he would receive by switching to gas permeable lenses, but he refused, insisting on soft lenses only. The patient did not have any information about his current soft contact lenses, which demonstrated 20/80 VA O.D. and 20/40 O.S. with good centration and rotation, but with 2+ deposits O.U. We decided to fit him with the CooperVision0p Frequency 55 Toric XR lens. After several trials and over-refractions, we finally found a suitable fit with VA of 20/40 O.D. and 20/30+2 O.S. He was satisfied with his vision and will be followed every six months. Although this case is unusual in that the discrepancy between the soft contact lenses and the glasses is large (usually there is not so much of a difference), it illustrates how what looks good or bad on paper does not always correlate with the actual fitting. |
One criterion I use to determine if soft lenses are acceptable is that they should induce no scarring. Soft contact lenses sometimes lead to repeat corneal abrasions that can result in scarring. The other criterion I use is a best-corrected visual acuity of 20/40 or better (usually the legal driving acuity).
GP lenses. When soft lenses no longer work, early keratoconus patients may achieve adequate acuity with large-diameter GP lenses or aspheric lens designs. Often, the large diameter is needed to achieve sufficient centration and to obtain a good fit. Since keratoconus patients often wear their contacts all day, it is important to use lenses with high oxygen permeability to minimize the risk of neovascularization. Some materials I recommend include the Boston EO, Fluorex 700, or the Fluoroperm 90 or 151. The other major concern is wettability, for which lenses such as the Hydro2 and the new XO are excellent choices. More advanced cases may require a lens designed specifically for keratoconus, such as the Soper, McGuire or Rose K. These fitting sets and procedures are outlined later in the article.
Piggyback lenses. If a keratoconus patient is intolerant of GP lenses, a GP lens worn over a hydrogel lens is sometimes a viable option. This concept, referred to as a piggyback fit, works well for many patients. This works by first fitting the patient with a large-diameter soft lens with proper centration and movement. Keratometry readings are then taken of the new corneal surface, and a gas permeable lens is fit over top of the soft lens. Because the patient is wearing two lenses simultaneously, it is even more crucial to make sure both lens materials are highly oxygen permeable.
Surgery. Once all contact lens options have been exhausted, surgery may be needed to obtain adequate vision. Surgical options include simple lamellar keratoplasty, thermokeratoplasty or penetrating keratoplasty.3 Another option is Intacs, which can be implanted to enhance the stability of the cornea and achieve a more stable fit.
To qualify for surgery, patients vision should be poor enough to interfere with their ability to work or drive. Like cataract surgery, there is no magic number at which the keratoconus patient needs to undergo a corneal transplant. Often, the decision to proceed to surgery is based on the patients perception of his or her vision. One patient may find that he cannot do his job with 20/30 acuity, while another patient may be very satisfied with her 20/60 vision. If a patient has a very large area of thinning toward the peripheral cornea, if a stable contact lens fit is no longer possible, or if the patient has significant corneal neovascularization, surgery may be performed earlier than otherwise indicated by visual performance alone.
Because keratoconus patients may need a corneal transplant in the future, avoid lenses that might induce corneal neovascularization because this condition increases susceptibility to graft rejection.
GP Contact Lens Fits
Three of the common gas permeable keratoconus fitting sets I use are the Soper Cone, McGuire and Rose K. What follows is a brief discussion of these sets and a summary of my fitting process for keratoconus patients.
The set I use most often is the Rose K, so this set is what I will focus on; but the principles I discuss can be applied to any GP fitting set.
Soper Cone. This is a bicurve contact lens with a fitting approach based on sagittal depth. In this lens design, the vaulting effect of the lens increases as the base curve decreases for a given diameter. This change in the steep central posterior curvature is used to fit the cone. This design is based on the notion of avoiding the apical bearing that will ultimately lead to corneal scarring in keratoconus patients.
The Soper design consists of 10 lenses, designated by the letters A to J. There are three groups for a given diameter/optic zone relationship.4 A, B, C and D are designed for mild keratoconus, or K-readings of less than 48.00D in either corneal meridian. E, F and G are for moderate keratoconus, or K-readings of 48.00D to 54.00D in either corneal meridian. H, I, and J are for the advanced stage, or K-readings of 54.00D or greater.3
McGuire. This keratoconus lens system was introduced in 1978 and is a modification of the Soper design.4 It consists of three diagnostic lens sets, each formulated for the nipple, oval or globus type of keratoconus. The fitting strategy is aimed at achieving a three-point touch predicated upon the size of optic zone in relation to conical size. In this design, the optical zone sizes are varied from 6.0mm for the nipple cone to 6.5mm for the oval cone and 7.0mm for the globus. Each lens also incorporates a series of four peripheral curves, which are blended together to create an almost aspheric relationship. The secondary curve of the McGuire system is 0.5mm flatter than the central base curve. The third curve is 1.0mm flatter than the secondary curve. The fourth and final peripheral curve is 2.0mm flatter than the tertiary curve.4
Rose K. This is a lens design with complex, computer-generated peripheral curves based on data collected by Dr. Paul Rose of Hamilton, New Zealand. To achieve the ideal edge lift of 0.8mm, the lens incorporates three peripheral systems: standard, flat and steep. It is available in base curves of 4.75 to 8.00mm and diameters of 7.9 to 10.2mm. The design works by decreasing the optic zone diameter as the base curve becomes steeper. Toric curves are available on both the front and rear surface as well as peripherally, but they are rarely needed.
Rose K lenses are traditionally made in the Boston ES material, but I have found labs that make them in the Boston EO material, which provides the added benefit of increased oxygen permeability. The Rose K manufacturing process features a unique software program used to help cut and blend the multiple curves, which makes replacement lenses easy to reproduce.
A new version of this lens, the Rose K 2, is expected to be released later this year. This lens design incorporates a sophisticated parabolic section on the posterior surface to counteract spherical aberration.
Spherical aberrations occur because the focal points of light rays far from the principal axis in a spherical lens are different from the focal points of rays that pass through the center of the axis. In keratoconus, this is exaggerated further in the steep radius of curvatures. The rays of light passing through the edge of the lens bend more and focus slightly in front of those passing through the center. The spherical aberration is counteracted by varying amounts of eccentricity across the back optic zone diameter. The fitting regimen for Rose K 2 lenses is the same as for Rose K 1. If successful, these lenses will be a welcome addition to the arsenal.
Fitting Protocol
One of the most important things to do when starting a keratoconus fit is to identify the morphological shape of the cone and thus determine the stage of the condition. Shape is easily assessed when viewing the cornea with retro-illumination after dilation. The stage of the condition can be assessed by examining K-readings. If the mean K is less than 50.00D, the cone can be considered early stage. A mean K-reading from 50.00D to 55.00D is advanced, and one of greater than 55.00D is severe.
|
|
| An example of a steep fitting Rose K lens. |
For any new fit, instill a drop of anesthetic in each eye prior to lens insertion. Otherwise, patients tear excessively, causing the lens to sit low and the eye to yield abnormal fluorescein patterns. Apply the lens and let the patient sit in the waiting room for at least 20 minutes before evaluating the fluorescein pattern. As with all other lenses, look at the central area, the midperipheral area and the periphery. Be sure that the lens is located centrally when you are evaluating it. If the lens lags down, use upward pressure on the lower lid to improve centration when judging fit. If you can demonstrate a good fit, you can often improve centration by increasing lens diameter.
In determining the correct base curve, start with one equivalent to the steeper of the two K-readings. Remember that the mires are often irregular in keratoconus, so the K-reading may provide only a rough gauge of the trial lens. Once you have assessed the fit of this lens (it shouldnt be too steep), continue to flatten the base curve until you get the slightest amount of apical touch. If you have a difficult time discerning where the apex of the cone is, then the lens is too steep.
The patient is generally most comfortable and will attain the best acuity when the weight-bearing forces of the contact lens are distributed evenly on the corneathe so-called three-point touch. This means that there should be minimal bearing (touch) at the apex of the cone, as well as an area of bearing between the periphery of the lens and the intermediate zone of the cornea. By making sure the ring of touch is incomplete, you permit freshly oxygenated tears to the central cornea when the patient blinks. By making sure the bearing is minimal at the apex of the cone, you will decrease the risk of scarring the cornea.
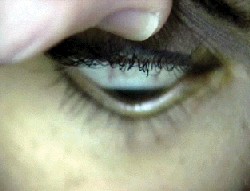 |
| Munson"s sign: bulging of the lower lid in downgaze. |
The trial lenses for the Rose K fitting set come in an 8.7mm diameter, which works for most patients. When evaluating diameter, the upper edge of the lens should hit the tarsal plate of the upper lid. If it does not, measure from the top of the lens to the bottom of the upper lid, and add that number to the overall lens diameter of the lens.
When doing the over-refraction start with 1.00D steps initially, and then refine with 0.50D and 0.25D increments as you get closer to the final prescription. Perform the final over-refraction in normal illumination to approximate normal light conditions and pupil size. If you find you cannot obtain adequate vision with a spherical overrefraction, try a spherocylindrical over-refraction. If a toric lens is ordered, the lens will be made 0.3mm larger than the diagnostic lens, and then a 0.3mm truncation is employed with 1 1/4 prism ballast to stabilize the lens. These toric Rose K lenses are not to be confused with bitoric GPs, which are generally contraindicated in keratoconus.
The criteria for a successful keratoconic lens fit are no different than for a standard fit. The lens needs to be comfortable enough to wear all day long, and the vision and post-wear biomicroscopy needs to be acceptable. The only difference is that acuity for the keratoconus patient may not be 20/20, even with contact lenses, but that is not a problem if the vision is satisfactory for the patients needs.
All keratoconus patients should return for follow-up at least every six months. Examine corneal surface integrity, evaluate lens fit, and look for changes in corneal topography. Remind them that keratoconus is a progressive disorder, and there is no way to predict when and if it will start to progress again. Also inform them they should schedule an office appointment immediately if they experience decreased vision, photophobia, decreased lens comfort, decreased lens wearing time, increased difficulty inserting or removing the contacts, or any similar symptoms. Such problems may indicate changes in the cornea that should be evaluated as soon as possible.
Common Fitting Problems
In our referral center, the single most common error in contact lens management of keratoconus is fitting too tight. The Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study found that the most common error was fitting excessively flat.
Your goal should be to achieve a good stable fit, while allowing for enough movement to provide adequate tear exchange. Otherwise, poor tear exchange leads to poor oxygen transmission to the cornea. Poor tear exchange also leads to a pooling of metabolic debris underneath the optic zone, which often shows up as dimple veiling and stipple staining around the base of the cone. This problem can be alleviated by decreasing the optic zone diameter, by flattening the existing secondary or peripheral curves, or by blending the junctions between the peripheral curves.5 Conversely, a lens fit too flat will move excessively and create discomfort. This can be fixed by steepening the base curve or by steepening the peripheral curves, which will decrease lens movement.
If you see coalesced staining of the apex of the cone, it is usually due to one of two reasons: the lens is too flat, or there are excessive deposits on the posterior lens surface. Apical staining may ultimately lead to corneal scarring, so this should not be permitted. If the fit is too flat, it should be steepened.
If the fit looks stable, have the patient remove the lens and observe the lens alone in the slit lamp.5 If the deposits are heavy, advise the patient to start a new lens and begin aggressive enzyming and a rigorous daily cleaning schedule. In some cases, it may be necessary for patients to enzyme as often as once or twice a week to keep deposits down.
Sometimes you will see small bubbles under the lens, which indicates a steep fit. This can usually be solved by flattening the base curve until you get the slightest apical touch, increasing the edge lift, or reducing the diameter/optic zone of the lens. If the bubbles persist, the lens can be fenestrated at the juncture of the optic zone and the secondary curve. Fenestration is not something you should routinely order on a lens, but it is a viable last resort.
If the lens is riding too low, you can improve centration by increasing the diameter or steepening the base curve. If you decide to change the diameter, the changes must be at least 0.3mm in most cases to see a clinical difference. If you see superior limbal staining or 3- and 9- oclock staining, reduce the diameter and/or increase edge lift.
Although the standard treatment of keratoconus involves contact lens fitting, encourage patients to use glasses for as long as possible. Particularly in the early stages of keratoconus, there is no need to hurry patients into contact lenses if they are happy with their vision. I find that a patient who has never worn hard lenses before must be highly motivated to get through the adaptation period of GPs.
The best way to attain this motivation is to keep the patient in glasses long enough so that he or she will notice the greatly improved vision obtained with GP lenses. If the patient is rushed into GPs and fails to notice a dramatic improvement, he or she may not be so willing to continue.
Before referring a patient for surgery, rethink your fitting strategy or send the patient to a colleague for a fresh approach. The vast majority of patients sent for surgery are denied that procedure and managed optically.
Dr. Gupta is the clinical director for The Center for Keratoconus at
Stamford Ophthalmology.