Who is Your Patient?This series explores how both innate and acquired elements of identity manifest in the clinic:
|
While race and ethnicity are often used interchangeably, race has to do with biological and physical features which generally cannot be hidden. Ethnicity refers more to cultural identification, language and customs adopted by people from a geographic region. Discussing race and ethnicity is fraught these days. Blame social media for stoking tribalism while amplifying hate and polarization. Profitability for the social media giants lies in optimizing user engagement by appealing to our paleolithic emotions. Users stay glued to these platforms when triggered into outrage, which amplifies culture wars. By first recognizing that social media latches onto the attention economy and succeeds when we are emotionally triggered, we can purposely begin a reflective, calm and meaningful discussion on the role of race and ethnicity in eye care.
Knowing a patient’s race and ethnicity can help optometrists with diagnosis and treatment when it comes from a place of compassion and empathy. What follows is a look into how race and ethnicity factor into diagnosing and treating select ocular conditions, including glaucoma, macular degeneration, diabetic eye disease, keratoconus and myopic degeneration. Finally, learn what to expect as healthcare moves from assessing patient race toward individual genetic make-up.
The Healthcare System
In law enforcement, racial and ethnic profiling is frowned upon, as it can discriminate against a minority population based on negative stereotypes. By comparison, in healthcare, physicians use race and ethnicity to predict disease risk and treatment efficacy. Classic medical examples include how cystic fibrosis is more common in white patients, specifically those of Northern European ancestry, and how sickle cell anemia affects predominantly Black people. In eye care, a retinal hemorrhage in a Black patient may signal sickle cell retinopathy. An Asian patient who complains of eye redness after drinking may be experiencing conjunctival hyperemia related to alcohol flush syndrome, as 60% to 80% of East Asians have a reduced ability to metabolize alcohol.1 Despite how race and ethnicity can help a clinician, doing so comes with certain dangers.
The shortcoming of using race and ethnicity to assess health risk is that it involves stereotyping and applying populational data to an individual. As clinicians, we must ask ourselves if this is always valid. Understandably, people of color may question race-based healthcare, particularly if it could lead to discriminatory care.
Indeed, there is evidence that racial and ethnic bias and stereotyping can lead to inequities in quality of care. For example, a 2016 study in Proceedings of the National Academy of Science investigated why Black Americans are systemically undertreated for pain compared with white Americans.2 The researchers found that half of medical students and residents surveyed believed that Black and white people are biologically different and that Black people are more tolerant of pain. This assumption was born from the slavery era when it was thought that Black people have thicker skin than white people, according to the researchers.
Black patients are almost four times more likely to suffer from kidney failure than non-Hispanic white patients.3 There is ongoing debate in nephrology on whether it is appropriate for doctors to routinely apply a “race correction” to their formula for the estimated glomerular filtration rate (GFR). This “correction” results in Black patients ending up with higher GFR values, suggesting better kidney function. In turn, this can contribute to worse kidney care for Black patients. For this reason, a growing number of medical institutions are abandoning race adjustment for estimated GFR. Critics say that lower health indicators among Black patients reflect the experience of being Black.
Finally, the COVID-19 pandemic has exposed differences in access to healthcare and preventive measures. People of color disproportionately bear the brunt of COVID-19 illness and mortality. However, Black and Hispanic people are not genetically more susceptible to coronavirus; rather, it appears that external factors hold greater influence.4 People of color commonly live in more crowded situations with less healthcare access, and a larger number tend to work in the food service and transportation industries where there is an increased contagion risk, a higher burden of cardiovascular and other chronic diseases and elevated exposure to stress related to violence and racism, all of which contribute to higher rates of COVID-19 illness and mortality.5
In 2020, the House Ways and Means Committee requested input from professional societies in healthcare to re-examine how race is misused within clinical care.6 In part, this was prompted by an article in the New England Journal of Medicine, which highlighted examples of race correction used in cardiology, nephrology, obstetrics and urology and considered how these clinical algorithms can perpetuate or even amplify race-based health inequities.7
Let’s now turn toward select eye conditions and the role race and ethnicity play in each.
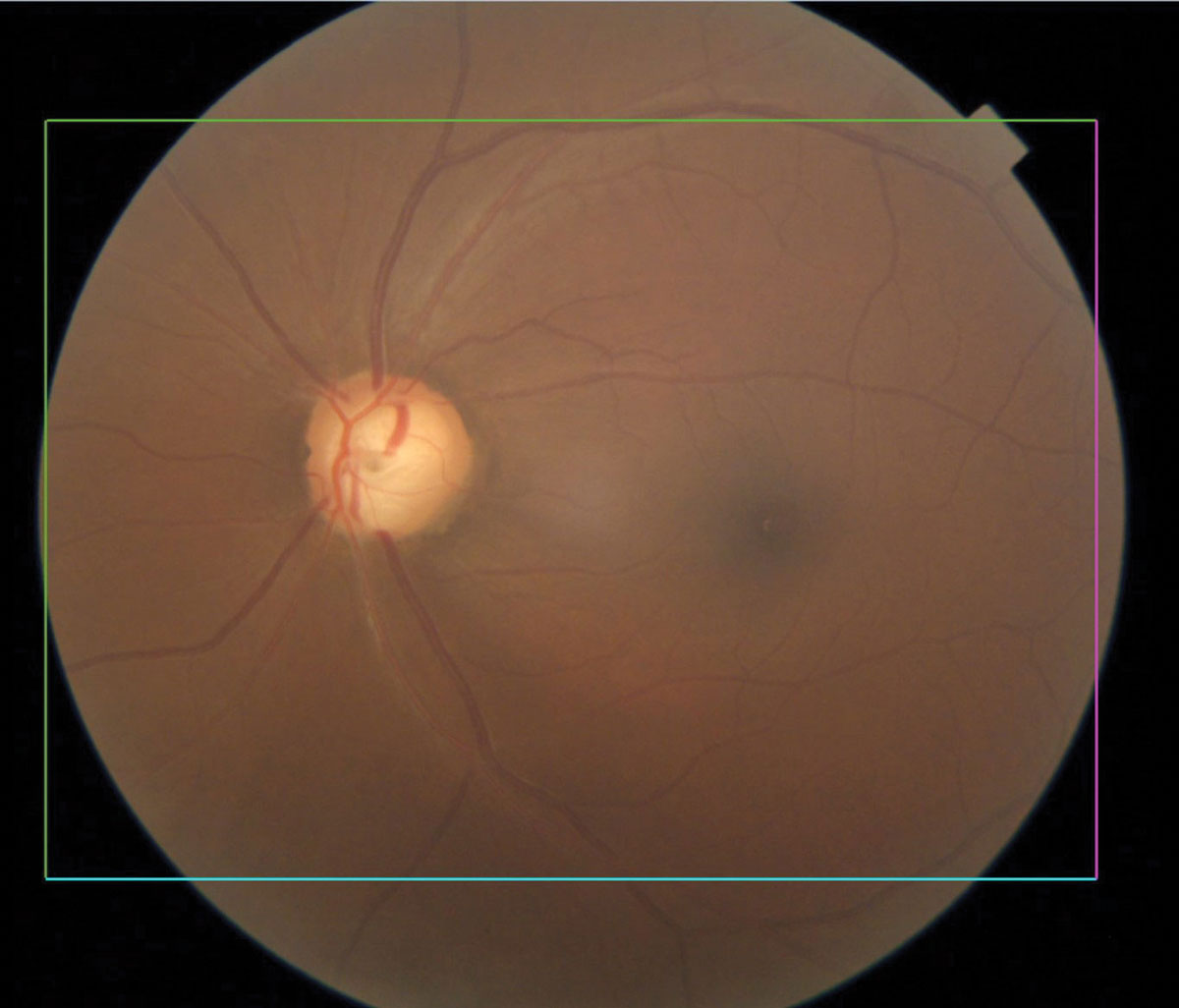 |
Glaucomatous optic nerve with inferior notching. Click image to enlarge. |
Glaucoma
The world’s leading cause of irreversible vision loss, glaucoma is a group of eye conditions that cause optic nerve damage, leading to blindness and visual impairment. Primary open-angle glaucoma shows increased prevalence and greater clinical severity in populations of African ancestry compared with those of European or Asian origin. It is six to eight times more likely to cause blindness and 15 times more likely to cause visual impairment in African Americans than Caucasians.8,9 The age-adjusted prevalence of open-angle glaucoma among African American residents of Baltimore receiving Medicare is 7.84% compared with 1.96% among their Caucasian counterparts.10
There is also a high prevalence of open-angle glaucoma among self-identified Latinos of primarily Mexican ancestry 40 years and older. According to the Los Angeles Latino Eye Study, in which 6,142 participants underwent a complete ophthalmologic examination at a clinical center, the prevalence of open-angle glaucoma was 4.74%.11
Despite the high prevalence of open-angle glaucoma among African Americans and Hispanics, the largest affected group in the United States is older non-Hispanic white women. However, this is expected to shift to Hispanic men over the next few decades.12
In terms of glaucoma detection, researchers recently suggested that normative databases for commercial OCTs must shift from predominantly Caucasian-based to include more ethnic-specific data. This would decrease the chances of glaucoma misclassification in different patient demographics whose OCT findings may vary.13
Importantly, a recent study published in Ophthalmology found that in a representative sample of Medicare beneficiaries with glaucoma, significant racial disparities exist in eye care utilization.14 After stratification by socioeconomic status, Black beneficiaries were less likely than whites to have outpatient follow-up and glaucoma testing but more likely to undergo procedural intervention for glaucoma. The authors concluded that this suggests systemic racism may independently drive these differences in Blacks, whereas disparities between Hispanic and Asian vs. white beneficiaries were largely explained by socioeconomic status.
Another recent paper, also in Ophthalmology, found that Black adults with glaucoma are significantly less likely to see an ophthalmologist or optometrist for glaucoma care, which may contribute to worse visual outcomes.15
Certain types of glaucoma are more common in specific groups. Vietnamese patients have a much higher prevalence of narrow angles and a greater risk of angle-closure glaucoma than white patients.16 Japanese American patients are at a much greater risk of normal tension glaucoma than white patients.17 Scandinavian patients are more likely to develop pseudo-exfoliative glaucoma.18
Glaucoma treatment can have different effects depending on a patient’s race. Prostaglandin analogs are the first-line pharmaceutical treatment due to their efficacy in reducing IOP, once-daily dosing and excellent safety profile.19 Curiously, the drug travoprost has greater efficacy among African Americans. In a large Phase III trial, travoprost 0.004% lowered IOP in African American patients by almost 2mm Hg more than non-African Americans.20 Furthermore, a higher percentage of African American patients responded to travoprost 0.004% and reached lower target IOPs than with either latanoprost 0.005% or timolol 0.5%.
On the other hand, certain glaucoma surgeries such as trabeculectomy may be less successful for Black patients due to an exaggerated healing response, suggested by a tendency for the conjunctivae of Black patients to contain more fibroblasts and conjunctival macrophages.21
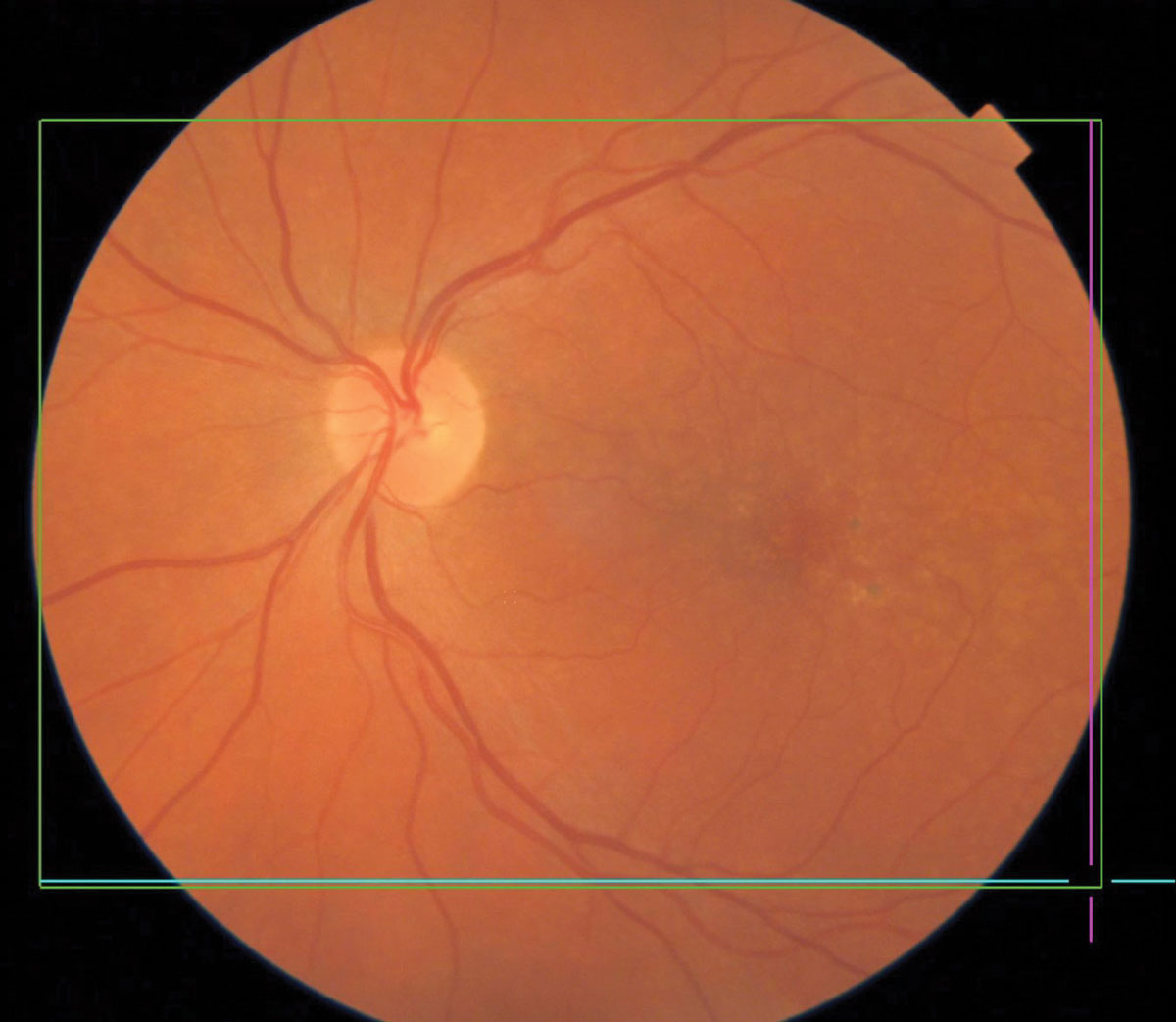 |
Pigmentary changes in dry AMD. Click image to enlarge. |
Macular Degeneration
AMD, a degenerative retinal disease impacting the elderly, arises from a complex relationship between genetics, age and external factors including smoking and diet. AMD is the leading cause of vision loss and blindness in Americans aged 65 and older.22
Older white patients are at the greatest risk of developing AMD. In 2010, 2.5% of white adults aged 50 and older had AMD, whereas 0.9% each of Blacks, Hispanics and people of other races had AMD.23
Multiple studies have reported a higher rate of AMD in white vs. Black patients. Pooled data from the Baltimore Eye Survey, Blue Mountains Eye Study, Beaver Dam Eye Study, Rotterdam Study, Melbourne Vision Impairment Project and Salisbury Eye Evaluation Project showed that in whites aged 80 and older, 16.4% of women and 11.9% of men had AMD.24 The same meta-review of pooled data for Black people from the Barbados Eye Study, Baltimore Eye Survey and Salisbury Eye Evaluation Project showed that female and male Blacks aged 80 and older had an AMD prevalence of 2.4% and 1.6%, respectively.
Caucasians with light irises may be more prone to AMD, according to one study.25 However, a meta-study found that it is not clear if this is always the case.26
A leading hypothesis is that the greater amount of melanin in RPE cells in Black populations may protect the RPE cells and Bruch’s membrane, either by acting as a free radical scavenger or absorbing high-energy wavelength light, and reduce drusen formation and pigmentary changes.27
Optometrists should anticipate older Caucasian patients’ concerns about AMD and take a proactive stance to discuss lifestyle modifications to minimize risk. In the same breath, this does not mean that optometrists should disregard the potential of AMD in other patient populations. It is possible that practitioners have bias in under-detecting and under-treating AMD in other racial groups. Studies have found that Black patients with AMD are 23% less likely to receive intravitreal anti-VEGF treatment and 18% less likely to have regular eye examinations compared with their white counterparts, although it is not known if this reflects clinician bias or other factors.28
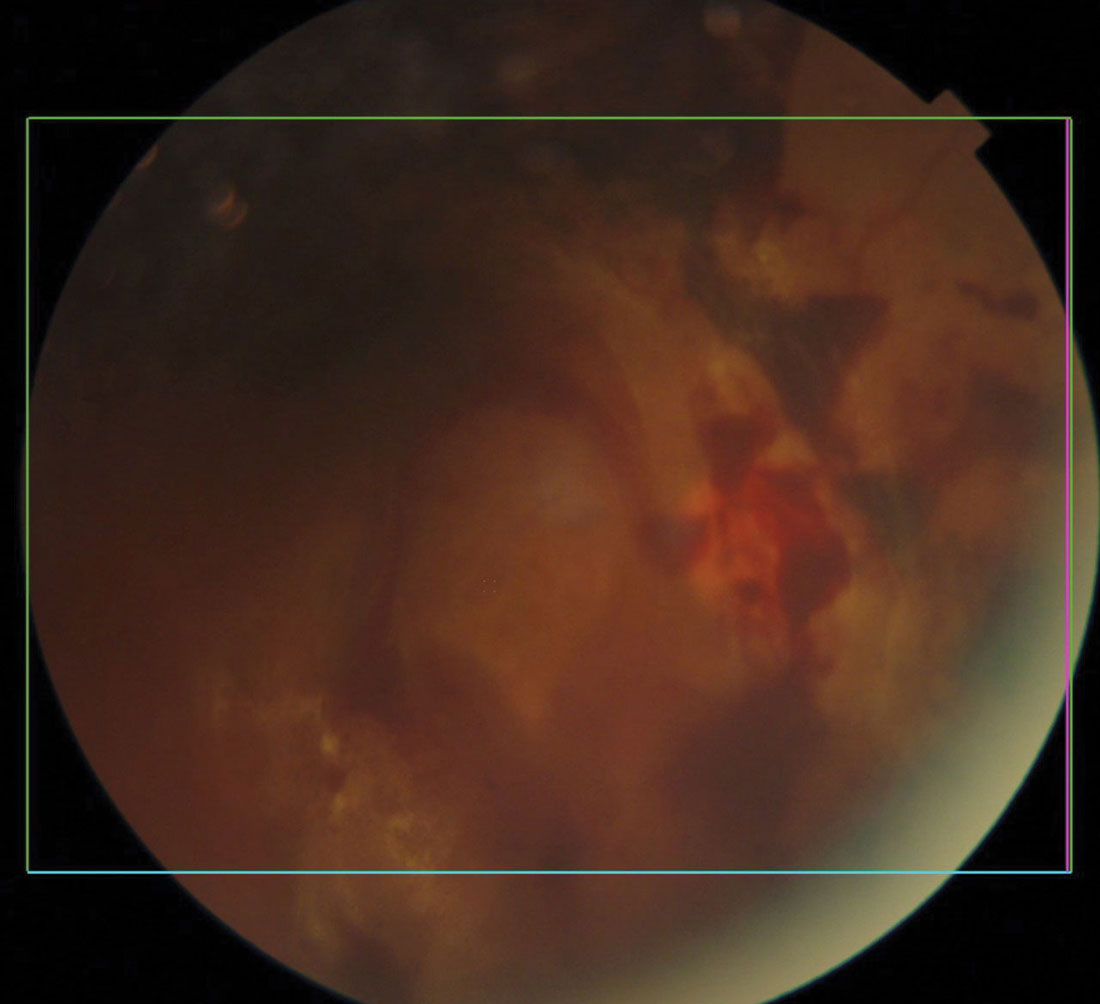 |
Diabetic retinal and vitreal hemorrhages. Click image to enlarge. |
Diabetic Eye Disease
Among the US population, numbers from 2018 estimated that 34.2 million people of all ages, or 10.5%, had diabetes.29 Diabetes is well-known to disproportionately impact racial and ethnic minorities.30 There are multiple factors that underlie these disparities, including biological, clinical, health system-related and social factors. Diabetes can increase the risk for cataract and glaucoma and lead to sight-threatening outcomes such as diabetic retinopathy.
The prevalence of diagnosed diabetes in US adults aged 18 and older is greatest among American Indians and Alaska natives (14.7%), followed by Hispanics (12.5%), non-Hispanic Blacks (11.7%), non-Hispanic Asians (9.2%) and non-Hispanic whites (7.5%).29
A retrospective study of 53,900 American Indians and Alaska natives with diabetes served by the Indian Health Services’ teleophthalmology program using ultra-widefield imaging found that 28.6% had diabetic retinopathy, 3% had diabetic macular edema and 3% had sight-threatening disease.31
The Veterans Affairs Diabetes Trial, involving 1,283 patients (~20% Hispanics and ~20% African Americans), found that severe diabetic retinopathy was more frequent in Hispanics and African Americans than in non-Hispanic whites.32 The authors indicated that these differences did not seem to be explained by age, duration of diabetes diagnosis, A1c or other standard risk factors. Although it might appear that Blacks are biologically at a greater risk for developing type 2 diabetes, a study published in 2017 in the Journal of the American Medical Association suggests that this may not be the case and that obesity is the primary culprit for the difference.33
Furthermore, among adult patients with diabetic macular edema who received intravitreal injections of bevacizumab, Black patients had a significantly lower likelihood of visual acuity improvement compared with white and Hispanic patients.34
As every optometrist knows, an eye exam may be the first indicator of systemic disease, including diabetes. Due to lower rates of racial and ethnic minorities seeking general healthcare, optometrists can play an important role in reinforcing why and when our patients need to see a physician to maintain their general health.
There are several online resources serving minority groups with elevated prevalence of diabetes. For example, the CDC has the Native Diabetes Wellness Program.35 The National Eye Institute and the National Eye Health Education Program have tip sheets encouraging clinicians to help African Americans and Hispanics/Latinos to reduce their risk of diabetic eye disease with the acronym TRACK: taking their medications, reaching and maintaining a healthy weight, adding physical activity to their daily routine, controlling their blood sugar, blood pressure and cholesterol, and kicking the smoking habit.36,37 To help communicate with native Spanish speakers, the National Eye Institute has Spanish language brochures on diabetic retinopathy available for download.38
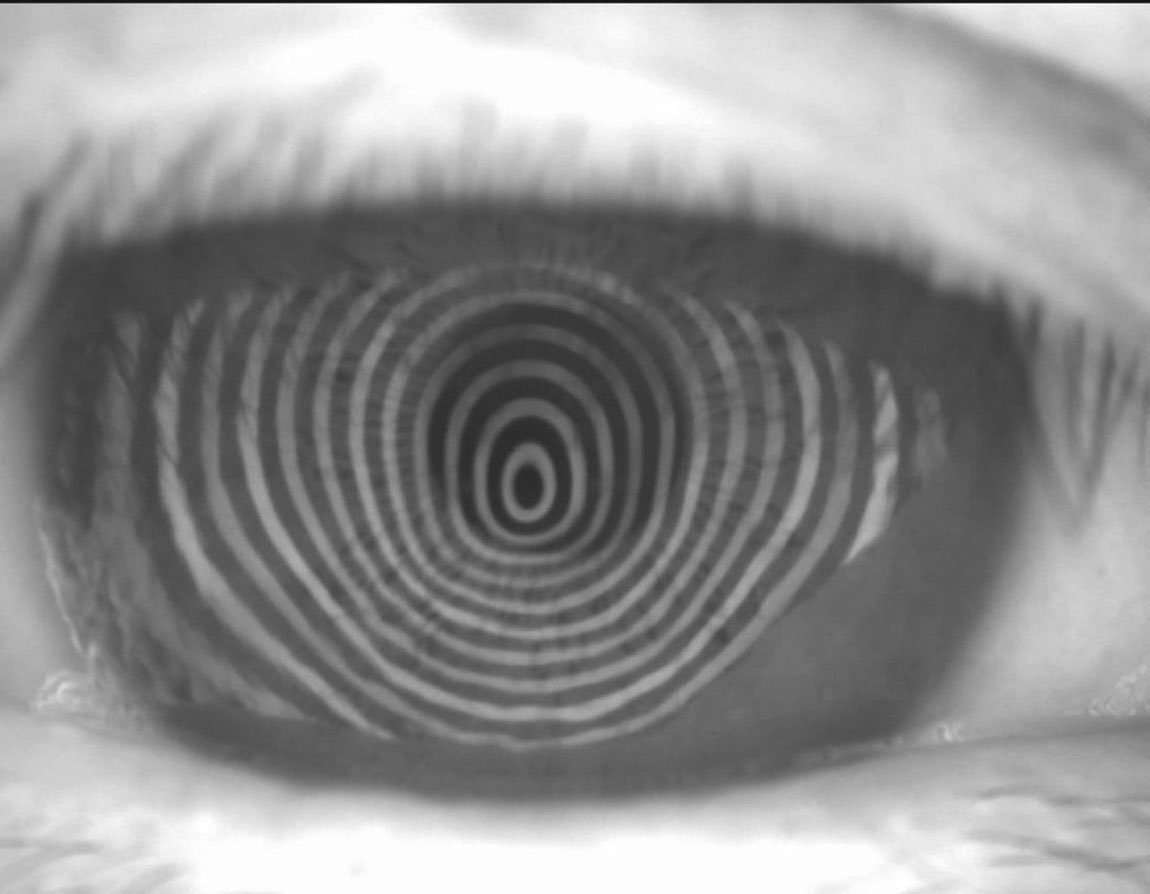 |
Irregular placido rings in corneal topography. Click image to enlarge. |
Keratoconus
While keratoconus comes nowhere close to the prevalence of glaucoma, macular degeneration or diabetic retinopathy, affecting about one in 375 individuals, it now gets a disproportionate amount of attention due to our ability to compellingly manage it with corneal crosslinking and scleral contact lenses.
Perhaps the most instructive study to date on race demographics and keratoconus was published in Ophthalmology in 2016.39 The authors looked at an entire US managed care network, identifying 16,053 beneficiaries with keratoconus. After accounting for confounding factors, Black patients had 57% higher odds of developing keratoconus, Latinos had 43% higher odds and Asian Americans had 39% lower odds compared with whites. Previous studies on race demographics and keratoconus did not include adequate populations of racial minorities to measure differences in risk.
The roughly 50% higher odds of keratoconus among Black and Latino patients was not previously described in the literature. This finding suggests value in increased screening for keratoconus in these populations. One of the leading manners of diagnosing keratoconus is during laser vision correction consultation. One study found that 8.59% of candidates seeking laser vision correction had keratoconus.40 Due to the high cost of elective refractive surgery, there may be fewer laser vision correction consultations among Black and Latino underprivileged populations and consequently fewer opportunities to diagnose keratoconus this way. This is similar to how these minority populations are disadvantaged by experiencing greater COVID-19 illness and mortality.41
Optometrists should be more inclined to suspect keratoconus in their Black and Latino patients and to order corneal topography when clinical findings include high and oblique astigmatism, reduced best-spectacle corrected visual acuity, lower measured IOP, steep keratometries, variable refraction outcomes, error messages on autorefraction and degraded image quality on retinal imaging and ocular coherence tomography. The stakes of early diagnosis are elevated due to the FDA’s 2016 approval of corneal crosslinking system iLink (Glaukos) and the opportunity to slow or arrest disease progression. However, even if keratoconus is diagnosed when stable, prompt identification is valuable due to the efficacy of scleral contact lenses in restoring functional vision and quality of life.42
The Ophthalmology study’s finding of lower risk of keratoconus among Asians differs from two previous studies out of the United Kingdom. The first UK study, with 338 keratoconus patients, found a four-fold increase in incidence of keratoconus among Asians.43 The second study (74 keratoconus patients) found over a seven-fold increase in incidence of keratoconus, with the authors noting that most of their Asian patients were from Northern Pakistan where the community has a tradition of consanguinity.44 Both UK studies did not perform multivariable regression modeling to account for confounding variables, which may explain the discrepancy with the Ophthalmology study.
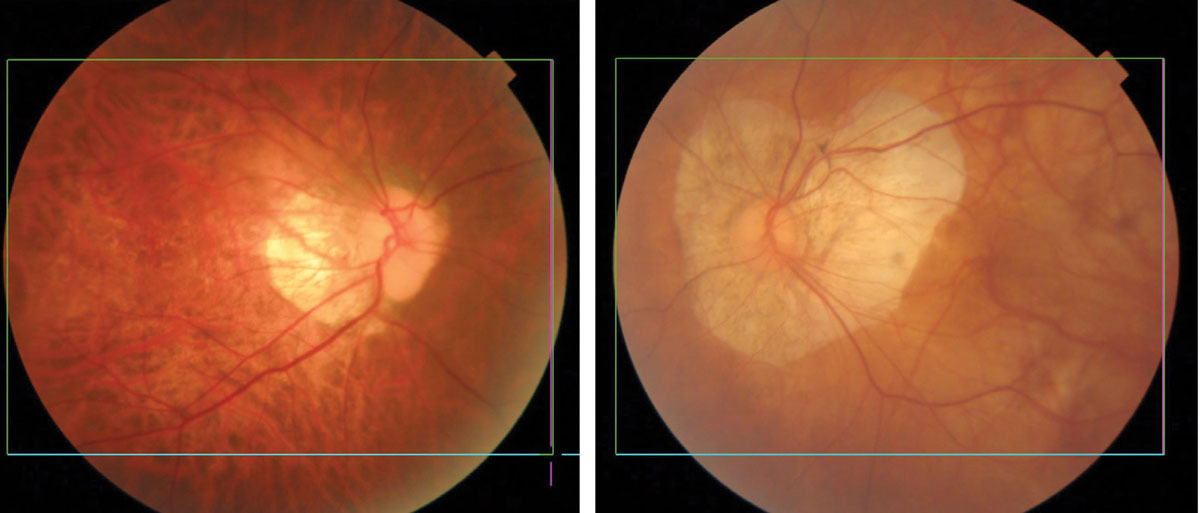 |
Myopic degeneration showing thinning and atrophy of the posterior pole. Eyes of Asian patients have more pigment (left) than eyes of Caucasians (right). Click image to enlarge. |
Myopic Degeneration
A literature review indicated myopia prevalence in school children ranges widely by geography (73% in East Asia, 42% in North America, 40% in Europe and 10% in Africa and South America).45 Genetic and environmental factors play a role in the increasing worldwide prevalence of myopia. Many clinicians are concerned about myopic progression potentially leading to vision loss due to myopic maculopathy and retinal pathology, including retinal detachment. Low levels of outdoor activity and prolonged near work are risk factors for myopia, according to recent studies.
Particularly among Chinese American parents, there is an elevated level of anxiety about their children’s myopia development. Anecdotally, practitioners with robust overnight corneal reshaping practices report that a disproportionate majority of these patients are Chinese American children. This ethnocentric observation may have underpinnings based on national policies in China.
In 1963, the Chinese Ministry of Education introduced daily eye exercises in all schools throughout China with the intent of reducing and preventing myopia.46 These exercises involve applying pressure to acupuncture points around the eyes, supposedly to improve blood circulation, reduce fatigue and minimize myopia development. Despite the longstanding national requirement for these exercises, their efficacy is questionable, with one study conducted over a two-year period not finding any statistically significant reduction in myopia onset or progression.47 In addition, some schools in China have used metal bars on student desks to prevent children from getting too close to their reading material and curtail myopia development.48
In 2018 Xi Jinping, president of China, urged his citizens to pay greater attention to the myopia epidemic in China.49 In May 2021, the country’s Ministry of Education revealed a five-year plan to reduce myopia, with school measures including limited digital screen time, paper-based homework and increased outdoor activities.50 Some of these measures are based on evidence, whereas others are based on popular beliefs. The collective effect of these policies is a cultural stigma surrounding myopia among the Chinese.
The heightened concern among Chinese American parents about their children’s myopia is common enough that practitioners should proactively discuss myopia management options as part of their treatment plan. An increasing number of optometrists view myopia management, which orbits around low-dose atropine, multifocal contact lenses and corneal reshaping, as a practice growth opportunity.
A Societal Shift
Healthcare seems to be moving in the right direction, identifying more appropriate treatment methods on an individual basis and increasing awareness of the need for such management options for better overall patient outcomes.
Personal genomics. A growing number of Americans identify as multi-racial. In the 2020 census, 9.6% of the total population was two or more races.51 With an increasing multi-racial population, the paradigm for using race and ethnicity to assess risk of eye disease becomes less clear. For example, how is an optometrist to assess glaucoma risk for a biracial Black and white woman? Instead, knowing the genetic make-up of a patient through genomic testing will become more relevant. The field of precision medicine is already making its mark on cancer treatment, using data-driven outcomes to guide the most effective management option based on an individual’s genetic make-up.52
Optometrists may envisage a day when glaucoma patients receive topical therapy or surgery according to their genomic data rather than self-reported race and ethnicity. There has already been a genome-wide association study (GWAS) of African ancestry populations to evaluate potential mechanisms of pathogenesis associated with primary open-angle glaucoma.53 GWAS has also been performed for exfoliation syndrome, which better elucidates the genetic basis of this condition and how secondary glaucoma may develop.54
Looking into the future, Avellino recently launched AvaGen, a genetic test claimed to definitively identify TGFBI gene corneal dystrophies including epithelial basement membrane, granular types 1 and 2, lattice type 1, lattice type 3a, Reis-Bucklers, Schnyder and Theil-Behnke. AvaGen also provides a risk assessment for keratoconus. The clinical validity and utility of many of these personal genomic tests is still unknown, but the hope is that they may allow for clinical interpretation with less clinician bias and stereotyping, with improved outcomes based on the most effective approach for an individual.55
Diversity recognition. Awareness of diversity, which also extends to religion, sexual orientation and gender identity, among other factors, is increasing. Millennials and Generation Z are much more likely to outwardly identify as LGBTQ than older generations.56 Similar to racial or ethnic risk for disease, there are health risks associated with the LGBTQ population. For example, there is mounting evidence that the LGBTQ population is at increased risk for cardiovascular disease compared with their cisgender heterosexual peers, posited to be driven primarily by psychosocial stressors.57 Cigar, cigarette and e-cigarette smoking is higher among transgender adults than adults whose gender identity corresponds with their birth sex.58 Recognizing increased risk can help better serve the patient, particularly when the clinician also acknowledges that there is individual variability.
Just like how an 80-year-old woman may wonder how a 25-year-old male optometrist can relate to her eye care needs, a similar dynamic may occur when a Black woman is examined by a white optometrist. The same can be said about other disparate characteristics between the patient and the clinician. Self-awareness is a good first step, but this doesn’t mean calling out obvious differences and making them an emphasis of the relationship.
Another key is recognizing that good care transcends racial and ethnic differences, language barriers, socioeconomic status and age. In each of these situations, human connection and trust can develop over shared emotions and feelings. Ultimately, humans are more in harmony with one another than we are different. We can still relate to each other based on commonalities while also celebrating our differences and the variety that they bring.
| 1. Gross ER, Zambelli VO, Small BA, et al. A personalized medicine approach for Asian Americans with the aldehyde dehydrogenase 2*2 variant. Annu Rev Pharmacol Toxicol. 2015;55:107-27. 2. Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. PNAS. 2016;113(16):4296-301. 3. Godoy M. KQED. Is it time for a race reckoning in kidney medicine? December 28, 2020. www.kqed.org/news/11852910/is-it-time-for-a-race-reckoning-in-kidney-medicine. Accessed December 9, 2021. 4. Richter R, Best J. Stanford Medicine. How accounting for race sabotages health treatment. May 10, 2021. stanmed.stanford.edu/2021issue1/race-based-medicine-sabotages-care.html. Accessed December 9, 2021. 5. Golden SH. Johns Hopkins Medicine. Coronavirus in African Americans and other people of color. April 20, 2020. hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/covid19-racial-disparities. Accessed December 9, 2021. 6. Ways and Means Committee. Ways and Means Committee issues request for information on the misuse of race within clinical care. September 17, 2020. waysandmeans.house.gov/media-center/press-releases/ways-and-means-committee-issues-request-information-misuse-race-within. Accessed December 9, 2021. 7. Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight — reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383:874-82. 8. Javitt JC, McBean AM, Nicholson GA, et al. Undertreatment of glaucoma among Black Americans. N Engl J Med. 1991;325:1418-22. 9. Muñoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Evaluation Study. Arch Ophthamol. 2000;118(6):819-25. 10. Tielsch JM, Sommer A, Katz J, et al. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA Ophthamol. 1991;266(3):369-74. 11. Varma R, Ying-Lai M, Francis BA, et al. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111(8):1439-48. 12. Vajaranant TS, Wu S, Torres M, et al. The changing face of primary open-angle glaucoma in the United States: demographic and geographic changes from 2011 to 2050. Am J Ophthamol. 2012;154(2):303-14. 13. Majithia S, Tham YC, Chun Yuen CC, et al. Retinal nerve fiber layer thickness and rim area profiles in asians: pooled analysis from the Asian Eye Epidemiology Consortium. Ophthalmology. November 29, 2021. [Epub ahead of print]. 14. Halawa OA, Kolli A, Oh G, et al. Racial and socioeconomic differences in eye care utilization among Medicare beneficiaries with glaucoma. Ophthalmology. October 6, 2021. [Epub ahead of print]. 15. Cheng BT, Tanna AP. Association of race and ethnicity with the frequency of outpatient glaucoma care. Ophthalmology. December 10, 2021. [Epub ahead of print]. 16. Nguyen N, Mora JS, Gaffney MM, et al. A high prevalence of occludable angles in a Vietnamese population. Ophthalmology. 1996;103(9):1426-31. 17. Pekmezci M, Vo B, Lim AK, et al. The characteristics of glaucoma in Japanese Americans. Arch Ophthalmol. 2009;127(2):167-71. 18. Arnarsson A, Damji KF, Sverrisson T, et al. Pseudoexfoliation in the Reykjavik Eye Study: prevalence and related ophthalmological variables. Acta Ophthalmol Scand, 2007;85(8):822-7. 19. Winkler NS, Fautsch MP. Effects of prostaglandin analogues on aqueous humor outflow pathways. J Ocul Pharmacol Ther. 2014;30(2-3):102-9. 20. Whitson JT. Travoprost--a new prostaglandin analogue for the treatment of glaucoma. Expert Opin Pharmacother. 2002;3(7):965-77. 21. Broadway D, Grierson I, Hitchings R. Racial differences in the results of glaucoma filtration surgery: are racial differences in the conjunctival cell profile important? Br J Ophthalmol. 1994;78(6):466-75. 22. CDC. Learn about age-related macular degeneration. www.cdc.gov/visionhealth/resources/features/macular-degeneration.html. Accessed December 9, 2021. 23. NIH. Age-related macular degeneration (AMD) data and statistics. www.nei.nih.gov/learn-about-eye-health/outreach-campaigns-and-resources/eye-health-data-and-statistics/age-related-macular-degeneration-amd-data-and-statistics. Accessed December 9, 2021. 24. The Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564-72. 25. Frank RN, Puklin JE, Stock C, Canter LA. Race, iris color, and age-related macular degeneration. Trans Am Ophthalmol Soc. 2000;98:109-15. 26. Chakravarthy U, Wong TY, Fletcher A, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31. 27. Age-related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology. 2000;107(12):2224-32. 28. Sloan FA, Brown DS, Carlisle ES, et al. Monitoring visual status: why patients do or do not comply with practice guidelines. Health Serv Res. 2004;39(5):1429-48. 29. CDC. National Diabetes Statistics Report 2020. Estimates of diabetes and its burden in the United States. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 9, 2021. 30. Golden SH, Brown A, Cauley JA, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors–an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2012;97(9):E1579-639. 31. Fonda SJ, Bursell SE, Lewis DG, et al. Prevalence of diabetic eye diseases in American Indians and Alaska Natives (AI/AN) as identified by the Indian Health Service’s National Teleophthalmology Program using ultrawide field imaging (UWFI). Ophthalmic Epidemiol. 2021;1-9. 32. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28(8):1954-8. 33. Bancks MP, Kershaw K, Carson AP, et al. Association of modifiable risk factors in young adulthood with racial disparity in incident type 2 diabetes during middle adulthood. JAMA Ophthalmol. 2017;318(24):2457-65. 34. Osathanugrah P, Sanjiv N, Siegel NH, et al. The impact of race on short-term treatment response to bevacizumab in diabetic macular edema. Am J Ophthalmol. 2021;222:310-7. 35. CDC. Native Diabetes Wellness Program. www.cdc.gov/diabetes/ndwp/index.html. Accessed December 9, 2021. 36. NIH. Educating African Americans about diabetic eye disease. www.nei.nih.gov/sites/default/files/nehep-pdfs/NEHEP_DED_AAI_TipSheet.pdf. Accessed December 9, 2021. 37. NIH. Educating Hispanics/Latinos about diabetic eye disease. www.nei.nih.gov/sites/default/files/nehep-pdfs/NEHEP_DED_HispanicLatinos_TipSheet.pdf. Accessed December 9, 2021. 38. NIH. Diabetic retinopathy: what you should know. www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/outreach-materials/la-retinopatia-diabetica-lo-que-usted-debe-saber-diabetic-retinopathy-what-you-should-know. Accessed December 9, 2021. 39. Woodward MA, Blachley TS, Stein JD. The association between sociodemographic factors, common systemic diseases, and keratoconus: an analysis of a nationwide healthcare claims database. Ophthalmology. 2016;123(3):457-65. 40. Althomali TA, Al-Qurashi IM, Al-Thagafi SM, et al. Prevalence of keratoconus among patients seeking laser vision correction in Taif area of Saudi Arabia. Saudi J Ophthalmol. 2018;32(2):114-8. 41. Alcendor DJ. Racial disparities-associated COVID-19 mortality among minority populations in the US. J Clin Med. 2020;9(8):2442. 42. Bergmanson JPG, Walker MK, Johnson LA. Assessing scleral contact lens satisfaction in a keratoconus population. Optom Vis Sci. 2016;93(8):855-60. 43. Pearson AR, Soneji B, Sarvananthan N, Sandford-Smith JH. Does ethnic origin influence the incidence or severity of keratoconus? Eye (Lond). 2000;14(Pt 4):625-8. 44. Georgiou, Funnell CL, Cassels-Brown A, O-Conor R. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asians and white patients. Eye(Lond). 2004;18(4):379-83. 45. Grzybowski A, Kanclerz P, Tsubota K, et al. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol. 2020;20(1):27. 46. Wang H, Qian Y, Congdon N, et al. Effect of Chinese eye exercises on change in visual acuity and eyeglasses wear among school-aged children in rural China: a propensity-score-matched cohort study. BMC Complement Med Ther. 2020;82:20. 47. Kang MT, Li SM, Peng X, et al. Chinese eye exercises and myopia development in school age children: a nested case-control study. Sci Rep. 2016;6:28531. 48. Inventor Spot. Anti-myopia school desks “bar” Chinese students from nearsightedness. inventorspot.com/articles/antimyopia_school_desks_bar_chinese_students_nearsightedness. Accessed December 9, 2021. 49. Binagwaho A. CNN. How lessons from Rwanda could help China fix its myopia crisis. November 15, 2018. edition.cnn.com/2018/11/14/health/china-myopia-rwanda-africa-intl/index.html. Accessed December 9, 2021. 50. Gov.cn. China launches 5-year campaign against youth myopia. May 11, 2021. english.www.gov.cn/statecouncil/ministries/202105/11/content_WS609a1cc4c6d0df57f98d9565.html. Accessed December 9, 2021. 51. United States Census Bureau. data.census.gov/cedsci/table?q=United%20States&tid=DECENNIALPL2020.P1. Accessed December 9, 2021. 52. NIH. Cancer genomics overview. www.cancer.gov/about-nci/organization/ccg/cancer-genomics-overview. Accessed December 9, 2021. 53. Hauser MA, Allingham RR, Aung T, et al. Association of genetic variants with primary open-angle glaucoma among individuals with African Ancestry. JAMA Ophthalmol. 2019;322(17):1682-91. 54. Aung T, Ozaki M, Lee MC, et al. Genetic association study of exfoliation syndrome identifies a protective rare variant at LOXL1 and five new susceptibility loci. Nat Genet. 2017;49(7):993-1004. 55. NIH. Regulation of genetic tests. www.genome.gov/about-genomics/policy-issues/Regulation-of-Genetic-Tests. Accessed December 9, 2021. 56. HuffPost. Sieczkowski. 20% Of millennials identify as LGBTQ, according to new GLAAD study. March 30, 2017. www.huffpost.com/entry/20-percent-millennials-lgbtq-glaad-study_n_58dd140be4b05eae031d8f9c. Accessed December 9, 2021. 57. Caceres BA, Streed Jr CG, Corliss HL, et al. Assessing and addressing cardiovascular health in LGBTQ adults: a scientific statement from the American Heart Association. Circulation. 2020;142(19):e321-32. 58. Buchting FO, Emory KT, Scout, et al. Transgender use of cigarettes, cigars, and e-cigarettes in a national study. AJPM. 2017;53(1):e1-7. 59. Pivnick EK, Rivas ML, Tolley EA, et al. Interpupillary distance in a normal Black population. Clin Genet. 1999;55(3):182-91. |


