24th Annual Surgery ReportFollow the links below to read other articles from annual update on surgery: Corneal Compromise: How to Assess the Risk of Post-LASIK Ectasia Understanding the Role of IOL Optics in Postoperative Vision Complaints Excise and Conquer: Adding Minor Surgical Procedures to the Optometric Office |
Ocular surface disease (OSD), including dry eye disease (DED), is among the most common postoperative complaints and should be addressed prior to any ocular surgery. Since patients are typically asymptomatic, it falls on the optometrist to evaluate all patients for signs of OSD. If any are found, optometrists should take the lead in treating and managing it prior to any procedure.
This article reviews how to spot signs of the myriad conditions that fall under the OSD umbrella—including DED, lid and lash conditions and allergies. It includes details about how these disease states can be unveiled using modern imaging technologies. Finally, it will offer treatment protocols designed to improve patients’ postoperative ocular surface outcomes.
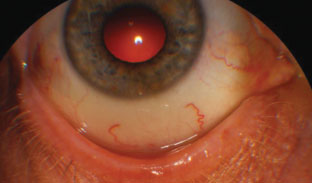 |
| This patient’s blepharitis is caused by either bacteria or Demodex—a mite infestation—on the lid. Resolution of either one is necessary before surgery. |
Ocular Surgery: Friend or Foe?
Refractive surgery techniques have evolved from incisional (RK) to surface ablation (PRK) to microkeratome and then femtosecond laser–assisted flap creation (LASIK) to small-incision lenticule extraction (SMILE). As these procedures evolved, the complications that affect the ocular surface have typically improved. According to a recent study, tear film dysfunction was identified as the most common reason for referral to a tertiary eye clinic following refractive surgery.1
Research shows LASIK has an impact on tear production and, therefore, could aggravate any underlying ocular surface issues in patients with moderate-to-severe DED.2 Photorefractive keratectomy (PRK) may need to be considered in this subset of patients because it causes less progression of DED. SMILE—currently only an option for patients who are myopic (with or without astigmatism)—is a minimally invasive approach to refractive surgery due to the lack of flap creation and may better preserve the corneal integrity and nerve density compared with LASIK.3
Cataract surgery has evolved over the years and provides excellent visual outcomes when the ocular surface is pretreated. Treating patients’ OSD and DED also helps to decrease the severity of postoperative symptoms. Research shows that corneal denervation persists up to three months with phacoemulsification.4 A recent study found that perioperative ocular parameters, such as high ocular surface disease index (OSDI) scores at baseline, low tear break-up time, low meibomian gland orifice obstruction scores and increased meibomian gland dropout one month after surgery were all risk factors for persistent dry eye symptoms after cataract surgery.5 As clinicians, we must be attuned to the variety of treatment options available and tailor them to individual patients.
Dry Eye Disease
DED is one of the most prevalent conditions in the United States, and perhaps one of the most commonly encountered diagnoses seen on a daily basis. According to a recent article based on weighted estimates, approximately 6.8% of American adults are projected to have DED.6,7 The prevalence of dry eye increases with age and is more predominant in women than men.6 The etiology of the condition is much more complex; however, we know that ocular surface inflammation is a key component of DED.6 Ocular disease, infection, or autoimmune conditions can cause chronic inflammation, and environmental exposures can exacerbate it.8
DED is classically divided into aqueous deficient dry eye (ADDE) and evaporative dry eye (EDE) with overlap occurring between the two.6 The two primary mechanisms that contribute to DED are tear hyperosmolarity and tear film instability.6 Hyperosmolarity results in an inflammatory cascade that damages the ocular surface and releases inflammatory mediators into the tears.6 Both ADDE and EDE are associated with tear hyperosmolarity.6 Tear film instability can arise secondary to tear hyperosmolarity, or can be the initiating event in the disease process.6 A reduced lipid layer in meibomian gland dysfunction can also cause tear film instability.6
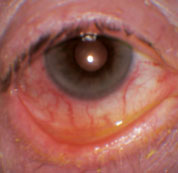 |
| Ocular rosacea, as seen here, can lead to dry eye and blepharitis issues that complicate surgery. |
DED Signs and Symptoms
The patient’s systemic history is vital to a proper diagnosis—and that includes any medications being taken. Tear deficiency causes dryness, red eyes, irritation, burning or foreign body sensation, excessive or lack of tearing, itching, light sensitivity, blurred vision, contact lens intolerance and eyestrain.9 DED may be associated with no signs and only symptoms or vice-versa.6
Uncovering signs and symptoms requires the use of various diagnostic tools. Questionnaires such as the OSDI are designed to detect patients with symptoms of DED prior to examination. Fluorescein stains may help identify signs and fluorescein dye can help evaluate tear break-up times. Lissamine green or rose bengal stains are used to evaluate the conjunctiva. Schirmer (and phenol red thread) testing may also provide information. Tear osmolarity testing can assess the osmolarity of the tears—a key sign of DED. Abnormal values are greater than 300mOsm/ml (or greater than 8mOsm/ml of inter-eye difference).10
Matrix metalloproteinase-9 (MMP-9) testing also shows a high sensitivity and specificity in diagnosing OSD. This test is measured using InflammaDry (Quidel) and is considered positive if the concentration of MMP-9 measured in the assay is higher than 40 ng/mL.10
If it is determined during a preoperative examination a patient is experiencing early DED, preventative management should be initiated.
DED Therapies
Once diagnosed, treatment should be based on the underlying condition(s) and the disease severity. Three basic strategies exist for ADDE and EDE: increase the amount of tears on the ocular surface, decrease tear evaporation and augment the lipid content or lubricity of the tears.8
Topical, over-the-counter lubricants, gels and ointments—both preserved and non-preserved—is one option to enhance the tear volume and quality. However, some cases may call for punctal plugs or cautery, autologous serum and therapeutic contact lenses such as scleral lenses available.
The Tear Film & Ocular Surface Society Dry Eye Workshop II report’s Diagnostic Methodology report gives us a starting point to assess DED severity as well as the diagnostic tests to perform, but DED has no one size fits all treatment. The screening DEQ-5 or OSDI confirms that a patient might have DED and triggers the diagnostic tests of noninvasive break-up time, osmolarity and ocular surface staining with fluorescein and lissamine green. On initial diagnosis, ODs must exclude conditions that mimic DED with the aid of the triaging questions and assess the risk factors that may inform management options. Over-the-counter lubricants are typically reserved for mild signs and symptoms of DED and more aggressive therapy should be initiated as necessary.11
Two options include Restasis (cyclosporine, Allergan) and Xiidra (lifitegrast, Shire). Restasis is designed to help increase tear production by reducing ocular surface inflammation and directly affecting lacrimal gland function. Xiidra is designed to block the interaction of ICAM-1 and LFA-1, which are key mediators of dry eye inflammation.12 Short-term steroid use may also reduce inflammation.
Fish oil and flaxseed supplements—previously believed to enhance tear production and quality—failed to show such results in a controversial recent study in the New England Journal of Medicine.13 Some critics cite flaws in the methodology of this study, making conclusions difficult to draw.
DED patients must take lifestyle and environmental considerations into account to optimize their treatments. That may mean wearing protective eyewear, using a humidifier, reducing screen time and even increasing fluid intake while avoiding triggers, such as direct air flow from air conditioners, fans and heating units.
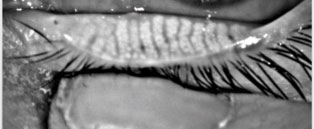 |
| This meibography image displays the glands of a patient healthy enough for surgery. Photo: Kambiz Silani, OD |
Lid Conditions
Not every compromised ocular surface is due to DED. Patients may be suffering from one (or more) of several conditions that DED therapies alone can’t address. For instance, research shows blepharitis in a whopping 60% of patients prior to surgery.14 It is the most common cause of cataract surgery cancellation, since it increases the risk of endophthalmitis.15 Patients with blepharitis will complain of itching, burning and crusting of the eyelids upon awakening.
Blepharitis is an eyelid inflammation that’s divided into two categories: anterior and posterior. Anterior blepharitis consists of bacteria, Demodex, or both, residing on the lid. Posterior blepharitis is more commonly referred to as meibomian gland disorder (MGD). Both can lead to telangiectasia, scarring of the lid margins and DED. Disruption of the anterior surface of the tear film from these conditions can cause surgical complications including infections and inflammation from causative bacteria, predominately Staphylococcus.16 Observation under the slit lamp is used for diagnosis of these conditions; however, noncontact meibography can be used to evaluate the integrity of the glands.17
Treatment includes cleaning the surface and glands with warm compresses and lid hygiene to reduce bacteria. A combination of steroids and antibiotic drops (or ointment) may decrease inflammation. Topical azithromycin can be used as well to decrease bacterial lipases, which degrade the normal meibum.18
Demodex—a type of mite that can cause anterior blepharitis—can be eliminated with preformulated wipes containing tea tree oil.18 You can determine the condition is resolved when expression of the meibomian glands provides a clear oil, free from milky toothpaste-like consistency.18 In office expression with thermal pulsation is available for severe or chronic obstruction.19 Systemic antibiotics such as doxycycline or minocycline have great results in clearing MGD, but should be used with caution due to contraindications and side effects.20
Treatment of these conditions before surgery can increase tear film quality and the accuracy of corneal topography and keratometry, creating better accuracy for intraocular lens alignment.14 It can also reduce the amount of bacteria in the tear film, reducing risk for infection or physical injury by the patient rubbing the eyelids.21
MGD can lead to morphological changes to the eyelid, which can increase dry eye symptoms indefinitely after cataract surgery.21 Stabilizing the tear film and eyelids can result in better surgical outcome including visual function, healing time and comfort.14
Ocular Allergies
Allergic reactions, a form of atopy, can affect the conjunctiva, cornea and lids.22,23 They damage the surface of the eye by creating chronic inflammation.24,25 During and after surgery, medications, and their preservatives, can exacerbate allergies.25 Current treatment methods aim to disrupt the allergic pathway. An important mast cell mediator is histamines, which aid in cell growth and extracellular matrix production.23 Release of tryptase by the mast cell in the conjunctiva activates the COX-2 pathway and fibroblast proliferation, aiding in the repair of the cornea after surgery or injury.23
Currently, ocular allergies are a contraindication for LASIK.25 Investigators have demonstrated that corneal haze and regression of myopia after PRK was more significant when allergic eye disease was left untreated.26 They found 12 months after surgery, patients with continued treatment of steroids and a mast cell stabilizer with allergic conjunctivitis, had significantly less corneal haze and less myopic regression.26 Another research team was able to show patients with allergic conjunctivitis had a more significant immune response after LASIK, increasing inflammation compared with a healthy individual, leading to a possible contribution of DED post surgery.25
Cyclosporine A can increase tear break-up time, according to the literature.24 In one study, it increased Schirmer’s Type I, decreased tear osmolarity and quickened the time frame for regaining corneal sensation.24 Cyclosporine A also has benefits for allergic eye disease, it inhibits interleukin (IL-II, IL-IV), and interferon gamma.24 It also inhibits lymphocyte proliferation with its interactions with T-cells, protecting goblet cells, which are essential to prevent DED.24
Treatment and management of ocular allergies includes removing the inciting factors and prescribing either antihistamines, mast cell stabilizers or a combination. More aggressive approaches, such as prescribing immunosuppressive therapies (i.e., steroids), cyclosporine A and in more serious cases treatment of secondary complications may be required depending on the duration and the severity of the reaction. More severe types of keratopathies may require surgical interventions or even amniotic membrane grafts. If left untreated, this chronic inflammation contributes to ocular surface damage and DED.22,25
Research does show it is possible to reach a good visual outcome using all methods of treatment, including immunosupressive therapies, surgical procedures and prosthetic lens placements.24
A Peek into the FutureOSD may be on the rise, but researchers are responding with a host of new pharmaceutical agents, diagnostic and treatment technologies and approaches to managing it. One new formulation, KPI-121 0.25% ophthalmic solution (Kala Pharmaceuticals) is undergoing phase III clinical trials in the Short Term Relief in Dry Eye (STRIDE) study for the short-term treatment of DED.1 Another, Cequa (cyclosporine A 0.09% ophthalmic solution, Sun Pharmaceuticals) incorporates nanomicellar technology to help increase tear production in patients with DED.1 Cequa was FDA approved earlier this year. Anterior segment optical coherence tomography is also being used to evaluate early ocular surface changes with epithelial thickness mapping, the first non-contact quantitative measure of the corneal epithelium and stroma.2 Additionally, researchers are developing better diagnostic methods, such as examining biomarkers for DED. For instance, research shows the PAX-6 protein (which can be evaluated in tears) is down-regulated in patients with Sjogren’s syndrome or clusterin.4
|
Ocular Rosacea
Fifty-eight percent to 72% of patients with rosacea—a chronic skin condition characterized by flushing, erythema, papules, pustules and telangiectasia of the central and periocular facial regions—develop the disease’s ocular subtype.26 While women are more commonly diagnosed with cutaneous rosacea, no gender predilection is known.27 Ophthalmologic findings typically present in patients between 40 and 59 years old.27
The condition is associated with inflammation, hyperkeratinization, hyperosmolarity and, ultimately, damage of the ocular surface.26,27
Clinical findings usually begin with eyelid abnormalities, such as lid margin erythema and telangiectasia, blepharitis, MGD, recurrent hordeola and chalazia.26-28 Tear film debris or reduced tear break-up time and interpalpebral hyperemia are common.28 Cicatricial conjunctivitis, fibrosis and symblepharon can affect the superior or inferior lids and conjunctiva.26 Corneal involvement occurs in up to 33% of rosacea patients in the form of superficial punctate keratitis, peripheral neovascularization, subepithelial marginal infiltrates, recurrent corneal erosions or even stromal ulceration and perforation, with the latter causing the patient pain and vision loss.26 Other forms of ocular inflammation such as iritis, episcleritis and scleritis are associated with ocular rosacea as well.26
These patients present with symptoms ranging from itching, burning, foreign body sensation, redness, tearing, photosensitivity, pain and lid swelling.26,28 Symptoms of ocular rosacea may wax and wane and correlate to individual triggers. Aggravating factors include exposure to sun or extreme temperatures, spicy foods, alcohol, exercise, menopause and emotional distress.26 Additonally, medications such as amiodarone and nasal steroids or topical irritants can exacerbate the condition.26
Managing the dry eye and blepharitis aspects of the disease starts with warm compresses, digital massage and eyelid scrubs with baby shampoo or tea tree oil followed by lubrication with artificial tears.26 Topical cyclosporine dosed twice daily has been shown to be advantageous due to the inflammatory component of the disease.28 Nutritional supplementation with fish oil and flaxseed may benefit patients and a few studies have shown favorable results with the use of oral omega-3 fatty acids.26
Oral tetracyclines given 500mg BID for two to three weeks can treat ocular rosacea.27,28 Doxycycline dosed 100mg QD to BID for six to 12 weeks is regularly prescribed.27,28 Another FDA-approved option is prescription of 40mg daily of doxycycline as a long-term treatment for ocular rosacea.27,28 If unsuccessful, or when tetracyclines may be contraindicated, oral azithromycin 500mg per day for two weeks can be an alternative therapy.27,28
Other management tools for ocular rosacea are dependent upon the signs and symptoms of each patient. LipiFlow (Tearscience) uses heat and stimulation of the eyelid to aid in meibomian gland outflow and has been helpful in addressing EDED, which usually accompanies ocular rosacea.27
Other management tools for ocular rosacea are to be determined by the signs and symptoms of individual patients. Excision and drainage of chalazia and the use of punctal plugs to alleviate associated dry eye complaints may be effective.
Patients who have any form of OSD before surgery will have OSD after surgery. It is important to diagnose ocular surface disease in patients being referred for surgery and then prepare, protect, rehabilitate and maintain a healthy ocular surface. This includes patient education as well as pretreating the ocular surface.
We must take an active role in determining who is at greatest risk of developing OSD and proactively treat patients prior to surgery to provide the best overall outcome.
Drs. Norris, Henney, Barnhart and Mandese are staff optometrists at the VA Health Care System in Orlando.
1. Patryn E, Vrijman V, Nieuwendaal C, et al. Indications for and outcomes of tertiary referrals in refractive surgery. J Refract Surg. 2014;30(1):54-61. 2. Lee J, Ryu C, Kim J, et al. Comparison of tear secretion and tear film instability after photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2000;26:1326-31. 3. Kobashi H, Kamiya K, Shimizu K. Dry eye after small incision lenticule extraction and femtosecond laser-assisted LASIK: meta-analysis. Cornea. 2017;36(1):85-91. 4. Gomes J, Azar D, Baudouin C, et al. TFOS DEWS II iatrogenic report. The Ocul Surf. 2017;15:511-38. 5. Choi Y, Park S, Jun I, et al. Perioperative ocular parameters associated with persistent dry eye symptoms after cataract surgery. Cornea. 2018;37(6):734-9. 6. Craig J, Nichols K, Akpek E, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276-83. 7. Farrano F, Fridman M, Stillman I, et al. Prevalance of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90-8. 8. Clayton J. Dry eye. N Engl J Med. 2018 Jun 7;378(23):2212-2223. 9. Valim V, Trevisani V, de Sousa J, et al. Current approach to dry eye disease. Clinical Rev Allerg Immunol. 2015;49:288-97. 10. Gupta P, Drinkwater O, VanDusen, K, et al. Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation. J Cataract Refract Surg. 2018;44(9):1090-6. 11. Wolffson, J, Arita, R, Chalmers, R, et al. TFOS DEWS II Diagnostic Methodology report. The Ocular Surface 2017; 15 (3)539-74. 12. Donnenfield E, Karpecki P, Majmudar P, et al. Safety of Lifitegrast Ophthalmic Solution 5.0% in Patients With Dry Eye Disease: A 1-Year, Multicenter, Randomized, Placebo-Controlled Study. Cornea. 2016;35(6):741-48. 13. Asbell P, Maguire M, Pistilli M, The Dry Eye Assessment and Management Study Research Group, et al. N-3 fatty acid supplementation for the treatment of dry eye disease. N Engl J Med. 2018;378:1681-90. 14. Luchs J, Buzzego C, Trattler W. Prevalence of blepharitis in patients scheduled for routine Cataract Surgery. Poster presented at ASCRS Symposium Cataract IOP and Rx Surgery. April 11, 2010. Boston MA. 15. Afsharkhamseh N, Movahedan A, Motahari H, Djalilian A. Cataract surgery in patients with ocular surface disease: an update in clinical diagnosis and treatment. J Ophthalmology. 2014;28(3):164–7. 16. Teweldemedhin M, Gebreyesus H, Atsbaha A, et al. Bacterial profile of ocular infections: a systematic review. BMC Ophthalmol. 2017;17(1):212. 17. Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmol. 2008;115(5):911-5. 18. Foulks G, Borchman D, Yappert M, Kakar S. Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: a comparative clinical and spectroscopic pilot study. Cornea. 2013;32(1):44-53. 19. Blackie C, Carlson A, Korb D. Treatment for meibomian gland dysfunction and dry eye symptoms with a single dose vectored thermal pulsation: a review. Curr Opin Ophthalmol. 2015;26(4):306-13. 20. Doughty M. On the prescribing of oral doxycycline or minocycline by UK optometrists as part of management of chronic Meibomian Gland Dysfunction (MGD). Cont Lens Anterior Eye. 2016;39(1):2-8. 21. Park Y, Hwang H, Kim H. Observation of influence of cataract surgery on the ocular surface. PLoS ONE. journals.plos.org/plosone/article?id=10.1371/journal.pone.0152460. October 3, 2016. Accessed November 15, 2018. 22. Kanski J. Clinical Ophthalmology: A Systemic Approach. 5th ed. Philadelphia: Butterworth-Heinemann, 2003. 23. Das S, Pasari A, Sangwan V. Vernal keratoconjunctivitis: culmination of management using immunosuppression, surgical, and prosthetic therapy over a quarter of a century. BMJ Case Reports. casereports.bmj.com/content/2016/bcr-2016-217759.abstract. November 8, 2016. Accessed November 15, 2018. 24. Hamada S, Moore t, Moore J, et al. Assessment of the effect of cyclosporine-A 0.05% emulsion on the ocular surface and corneal sensation following cataract surgery. Contact Lens and Anterior Eye. 2016;39(1):15-9. 25. Wilson D, Schutte S, Abel S. Comparing the efficacy of ophthalmic NSAIDs in common indications: A literature review to support cost-effective prescribing. Ann Pharmacother. 2015;49(6):727-34. 26. Wladis E, Adam A. Treatment of Ocular Rosacea. Surv Ophthalmol. 2018;63(3):340-6. 27. Vieira Ana, Höfling-Lima A, Mannis M. Ocular rosacea: a review. Arquivos Brasileiros de Oftalmologia. 2012;75(5):363-9. 28. Arman, Aysegul, et al. “Treatment of Ocular Rosacea: Comparative Study of Topical Cyclosporine and Oral Doxycycline.” International Journal of Ophthalmology, U.S. National Library of Medicine, 18 June 2015, www.ncbi.nlm.nih.gov/pmc/articles/PMC4458660/. |

