 An 82-year-old white male presented with a chief complaint of photophobia and glare in both eyes. His ocular history was remarkable for bilateral cataract surgery nine months earlier. His systemic history was significant for a triple heart bypass and prostate cancer. Additionally, he reported using Flomax (tamsulosin, Boehringer Ingelheim) for several years before his cataract procedure. The patient had no known allergies of any kind.
An 82-year-old white male presented with a chief complaint of photophobia and glare in both eyes. His ocular history was remarkable for bilateral cataract surgery nine months earlier. His systemic history was significant for a triple heart bypass and prostate cancer. Additionally, he reported using Flomax (tamsulosin, Boehringer Ingelheim) for several years before his cataract procedure. The patient had no known allergies of any kind.
Diagnostic Data
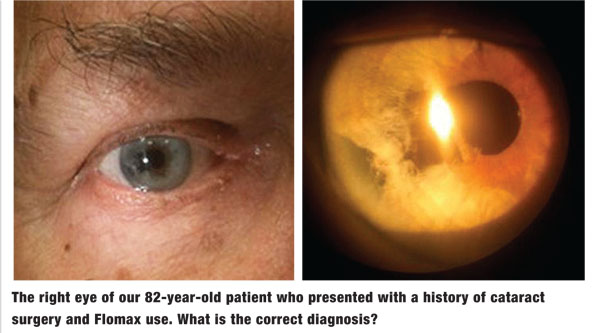
His best-corrected visual acuity measured 20/25 OD and 20/20 OS. His pupillary evaluation revealed no evidence of afferent defect. Extraocular muscle movements were full and unrestricted. There was no evidence of corneal pathology or anterior chamber reaction OU. Intraocular pressure measured 18mm Hg OU. The dilated fundus examination revealed normal and quiet grounds in both eyes. The pertinent clinical findings OD are illustrated in the photographs.
Your Diagnosis
How would you approach this case? Does the patient require any additional tests? What is your diagnosis? How would you manage this patient? What is the likely prognosis?
Discussion
The diagnosis in this case is significant tissue loss and corectopia secondary to intraoperative floppy iris syndrome (IFIS) following cataract surgery. It is well established that patients with a history of Flomax use are at increased risk for IFIS following cataract removal. Those with a history of Flomax use should undergo pupil measurements in bright and dim environments, keratometry and iris anomaly evaluation. IFIS is characterized by multiple clinical manifestations that frequently complicate cataract surgery, including poor preoperative pupillary dilation, billowing and prolapse of the iris stroma toward the phaco and incision site, and progressive intraoperative miosis.1,2
Even if the patient discontinues Flomax use months to years before surgery, the risk of IFIS remains elevated.1,3 Several pharmaceutical treatments or surgical accessories can help reduce the incidence and/or severity of IFIS, including:1-3
• Topical atropine or an intracameral injection of alpha agonists.
• Highly cohesive viscoelsatic devices (i.e., Healon 5, Abbott Medical Optics)
• Mechanical dilation implants (i.e., Malyugin Ring, MicroSurgical Technology).
Iris pigmentary loss secondary to cataract removal can induce visually significant glare. The extent of iris loss and overall tissue stability dictates the most appropriate reconstruction procedure.4 Typical surgical interventions include pupilloplasty or iris prosthesis. In pupilloplasty, prolene sutures are fixed to defect area in order to pull the iris edges together.4,5 In iris prosthesis procedures, a reconstruction implant is placed into the capsular bag or sutured into place with scleral fixation.4,5
Less invasive options include tinted contact lenses or lenses with hand-painted, artificial irides. Both cosmetic lens types have specific disadvantages, however. For example––tinted lenses do not effectively block light rays, and hand-painted lenses can be thick and uncomfortable.4
Considering our patient’s age, the amount of iris stromal pigmentary loss and his general reluctance to undergo a repairative surgical procedure, we decided that a cosmetic contact lens solution was the most appropriate option. Because of its unique construction and ability to match the patient’s natural iris color, we selected a Special Eyes Durasoft 3 (Alcon) colored contact lens for each eye. This lens features a conventional, gray-colored, single print with a gray underprint. The underprint ensures decreased light transmission, which minimizes the visual symptoms produced by the natural iris defects. A solid black underprint also is available; however, the gray underprint yielded a better aesthetic match in our patient.
Further, to minimize movement while still maintaining optimal corneal physiology, we fit the lens slightly steeper than the keratometry values indicated. Following the initial fitting, we ordered the patient’s lenses and informed him that they would arrive in approximately 10 business days. Once the lenses arrive, we will schedule the patient for a follow-up visit so we can observe and/or help improve his insertion and removal techniques.
1. Chang D, Braga-Mele R, Mamalis N, et al. Clinical experience with intraoperative floppy-iris syndrome. J Cataract Refract Surg. 2008 Jul;34(7):1201-9. 2. Chang D, Braga-Mele R, Mamalis N, et al. ASCRS White Paper: Clinical review of intraoperative floppy-iris syndrome. J Cataract Refract Surg. 2008 Dec;34(12):2153-62 3. Skorin L. How to avoid intraoperative floppy iris syndrome. Rev Optom. 2010;147(11):55-61. 4. Marques F, Marques D, Oetting T, et al. Absent iris tissue. Cataract Refrac Surg Today. 2007;33:25-30. 5. Srinivasan S, Yuen C, Watts M, et al. Endocapsular iris reconstruction implants for acquired iris defects: a clinical study. Eye (Lond). 2007 Aug;21(8):1109-13.

