 A 71-year-old white male presented as a new patient in
August 2009 for a diabetic retinal evaluation. He reported that another eye
care provider performed his last eye examination approximately six years
earlier, but I recognized him as a former patient and estimated that his last
eye examination at our office was in the early 1990s.
A 71-year-old white male presented as a new patient in
August 2009 for a diabetic retinal evaluation. He reported that another eye
care provider performed his last eye examination approximately six years
earlier, but I recognized him as a former patient and estimated that his last
eye examination at our office was in the early 1990s.
His current medications included metformin b.i.d. and glipizide q.d. for the treatment of non- insulin-dependant diabetes mellitus as well as 325mg aspirin q.d. The patient was first diagnosed with type 2 diabetes 12 years ago. He said that his HbA1c level measured 7.7% in spring 2009 and that his fasting glucose level the morning of the visit was 135mg/dL. He reported no known allergies to medications.
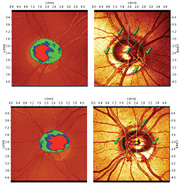 |
| 1, 2. The Moorfields Regression Analysis (MRA) measured his cup-to-disc ratio as 0.40 x 0.50 O.D. (top) and 0.60 x 0.60 O.S. (bottom), and confirmed aberrant neuroretinal rims in the inferior temporal regions O.U. (O.S.>O.D.) |
The patient’s best-corrected visual acuity was 20/20-1 O.D. and O.S., and 20/20 O.U. His pupils were equally round and reactive to light and accommodation, with no afferent pupillary defect. Slit lamp examination was essentially unremarkable, with the exception of bilateral dermatochalasis. Intraocular pressure measured 24mm Hg O.U. at 4:00 p.m. His anterior chamber angles, as estimated by Van Herick’s method, were grade 3 open O.U. Through dilated pupils, his crystalline lenses were characterized by mild nuclear sclerosis O.U. He demonstrated no evidence of a posterior vitreous detachment. His optic nerves were of average size, with a cup-to-disc ratio of 0.75 x 0.85 O.D. and 0.85 x 0.85 O.S.
Peripapillary atrophy was present temporally in each eye, and the inferior temporal neuroretinal rims were markedly thinned (O.S.>O.D.). His macular evaluation was characterized by several microaneurysms outside the foveal avascular zone O.U., and there was no evidence of clinically significant macular edema. There were scattered mid-peripheral microaneurysms and intraretinal dot-blot hemorrhages in both eyes—consistent with mild, non-proliferative diabetic retinopathy. There was no disc neovascularization in either eye. His peripheral retinal examination was unremarkable.
Given the suspicious appearance of his optic nerves, we asked him to return in two weeks for a full glaucoma work-up. At the work-up, his IOP measured 23mm Hg O.U. at 3:00 p.m. Pachymetry readings were 624µm O.D. and 620µm O.S. Threshold white-on-white visual fields were reasonably accurate and showed no glaucomatous defects in either eye. Gonioscopy demonstrated grade III-IV open angles O.U., with 2+ pigment in the trabecular meshwork in both eyes. There was no neovascularization of the iris or angles in either eye.
We obtained optic nerve stereo photographs as well as a baseline Heidelberg Retina Tomography-3 (HRT-3, Heidelberg Engineering). The Moorfields Regression Analysis (MRA) measured his cup-to-disc ratio as 0.40 x 0.50 O.D. and 0.60 x 0.60 O.S., and confirmed aberrant neuroretinal rims in the inferior temporal regions O.U. (O.S.>O.D.) (figures 1 and 2). We diagnosed the patient with open-angle glaucoma and started him on 1 drop h.s. Travatan Z (travoprost, Alcon) O.U.
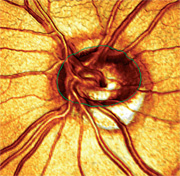 |
| 3. A 3-D image of the patient’s left eye, which shows severe thinning of the inferior temporal optic nerve as well as a minimal amount of optic nerve tissue within the confines of the optic canal. This analysis more closely approximates my initial grading of the cup-to-disc ratio (0.85 x 0.85) than the MRA measurement (0.60 x 0.60). |
I discussed the findings with the patient after determining that he had untreated open-angle glaucoma with significant optic nerve damage (O.S.>O.D.). During the discussion, he stressed that his previous eye care provider also raised the question of glaucoma, but the patient seemed convinced that his IOP simply “ran high.” Hearing that, I considered that the previous eye doctor diagnosed the patient as an ocular hypertensive with thick corneas and decided to observe him. (The patient’s records at our office from the early 1990s were purged several years earlier.) In this case, the patient’s optic nerves clearly showed evidence of significant glaucomatous optic neuropathy. But, he actually does have rather thick corneas with a slightly elevated IOP. Without a critical view of the optic nerves, an eye care physician may judge him to be an ocular hypertensive with thick corneas––someone with a statistically low risk of converting to glaucoma. Though the Ocular Hypertension Treatment Study (OHTS) suggested that patients with thicker corneas (greater than or equal to 588µm) are significantly less likely to develop glaucoma, there are patients with thick corneas and moderately high IOP who do, in fact, have optic nerve damage that requires timely medical intervention.1,2
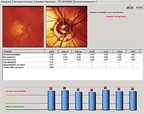 |
| 4. Subsequent analysis of his left optic nerve with the Glaucoma Probability Score (GPS) confirmed significant aberration of multiple regions. |
Though I look at a significant number of glaucomatous optic nerves every day, there were aspects of this patient’s neuroretinal rims that influenced my decision to label him a moderately advanced glaucoma patient, rather than a patient with early disease. My analysis of the cup-to-disc ratios was more indicative of damage than the MRA analysis (figures 3 and 4). Though the MRA demonstrated mild optic nerve abnormalities, the Glaucoma Probability Score analysis of the same data showed an optic nerve with substantially more damage––a finding that correlated well with my clinical evaluation of the optic nerves and overall impression.
The bottom line: Relying too much on one piece of data in the glaucomatous puzzle, such as central corneal thickness, can lead to false conclusions. While many factors need to be considered in the context of glaucoma, the most critical detail is a thorough slit lamp evaluation of the optic nerve. Spend your time evaluating the optic nerve, and your diagnostic acumen will increase proportionately. As for our patient, we scheduled him for a three-week follow-up to monitor the effectiveness of the Travatan regimen.
1. Brandt JD, Beiser JA, Kass MA, Gordon MO. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS). Ophthalmology. 2001 Oct;108(10):1779-88.
2. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002 Jun;120(6):714-20; discussion 829-30.

