Most likely you have encountered cases of optic neuropathy in your practice over the years, especially considering that it is one of the more common causes of acute vision loss or majorly impaired vision. While optic neuropathy refers to optic nerve damage from any cause, it has many specific subtypes—and they are not all created equal.
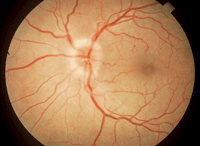
1. The optic nerve will be edematous in about 35% of optic neuritis cases.
In this article, we focus on acute optic neuropathies, which typically present with a very sudden decrease and/or loss of vision in one eye secondary to unilateral optic nerve swelling with a defined afferent pupillary defect (APD). There will be an associated unilateral visual field defect, and the onset typically is very dramatic to the patient.
The most common acute optic neuropathies include ischemic optic neuropathy (ION), optic neuritis and trauma. In patients ages 50 and up, acute anterior ischemic optic neuropathy (AION) is the most common presentation.1 AION is divided further into non-arteritic (NAION) and arteritic (AAION). AAION is the ocular manifestation of giant cell arteritis (GCA), also known as temporal arteritis. Another variant of ION is posterior ischemic neuropathy (PION), an infarction of the posterior portion of the optic nerve(s). To provide the most effective treatment for patients, it is crucial to understand each of these subtypes, because the underlying cause, disease progression and management varies in each case.1
Perhaps the most common neuropathy presentation is optic neuritis. Optic neuritis must be considered in cases presenting with visual disturbances and a unilateral swollen optic nerve in patients younger than age 45. Time is on your side in the diagnosis and treatment of optic neuritis and NAION, but it is your enemy in AAION.
Patients with GCA potentially can go blind in a matter of days—not to mention their increased risk for stroke or myocardial infarction. It is preventable with timely diagnosis and treatment, and for that reason, AAION is considered an ophthalmic emergency. This is why it is critical to clinically differentiate between AAION and NAION (the differential diagnosis of NAION is made via the exclusion of AAION).
In some instances, cost-effective laboratory testing and clinical observation can be used to properly diagnose these optic nerve diseases, without the need for radiology in typical, acute presentations. In atypical presentations, neuroimaging will be indicated. Neuroimaging also will be required in the differential diagnosis of multiple sclerosis (MS) in cases of optic neuritis.
Optic Neuritis
The classic presentation of optic neuritis is a sudden, unilateral loss of vision, with the distinct symptom of pain on eye movement. Typically, the patient is less than 45 years of age. The patient usually has a relative APD, color desaturation, brightness reduction and a unilateral central visual field defect.
figure 1).2 The majority (65%) will have no visible optic nerve edema initially—these cases are known as retrobulbar optic neuritis.2 Optic neuritis improves over three to six weeks without treatment. The first treatment should not be oral corticosteroids, per the protocol outlined by the Optic Neuritis Treatment Trial (ONTT).2
Because the risk of demyelinating disease is high in cases presenting as optic neuritis, patients should receive an MRI with gadolinium. If the MRI is positive for multiple plaque lesions (unidentified bright objects and “railroad tracking”), the patient most likely has MS and can be advised to consult a neurologist regarding treatment options. Image manipulation with fluid attenuated inversion recovery will assist in improved lesion isolation.
Patients with an atypical presentation of optic neuritis, unusual age, chronic occurrences as opposed to acute onset, lack of pain upon extraocular muscle movement and lack of improvement in three weeks potentially have an intracranial mass and should undergo appropriate consultation and neuroimaging.
Non-Arteritic Anterior Ischemic Optic Neuropathy
NAION is caused by a lack of optic nerve profusion or embolic disease that affects the arteries/arterioles that are supplying the optic nerve. Typically, NAION presents as a sudden, unilateral, painless loss of vision in patients age 50 or older. The patient usually has associated vasculopathic risk factors, including—but not limited to—hypertension, arteriosclerotic diseases, diabetes and nocturnal hypotension.
The patient often wakes up with a profound visual disturbance in one eye. Ophthalmoscopically, the patient presents with either segmental or total disc edema (
figure 2). Splinter hemorrhages of the optic nerve are common. Pupillary testing will show a frank APD in the affected eye. There will be a unilateral visual field defect, which can be superior or inferior, with a classic altitudinal hemianopsia (
figure 3). Some patients will have an inferior nasal visual field defect in the affected eye.
Patients with NAION will have a normal C-reactive protein and erythrocyte sedimentation rate. They also lack the typical constitutional symptoms that are often associated with GCA. Atypical clinical findings include: younger age, lack of acute onset, visual field defect that does fit with typical AION, proptosis, ocular motor paralysis, bilateral findings, and many of the other systemic signs and symptoms of occult neurologic disease. These patients will require consultation with neuroimaging of the brain and orbits with gadolinium enhancement. Bilateral disc edema is papilledema, until proven otherwise with neuroimaging for an intracranial mass. A patient with a swollen optic nerve in one eye and a visual field defect in the other eye is also a brain tumor, until proven otherwise.
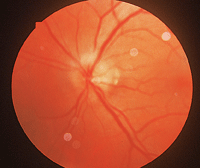
2. Ophthalmoscopically, the patient presents with either segmental or total disc edema.
There is no proven effective treatment for NAION at this time.3 Some suggested treatments include oral steroid agents to lower intraocular pressure, asprin therapy and intraocular anti-VEGF treatment.4 The majority of these eyes will progress to optic atrophy with a poor visual outcome (
figure 4). Some patients will have vision improvement in two weeks, with gradual vision improvement up to six months. If oral steroids are used in the treatment of NAION, the steroid must be started within 14 days of onset.4
Fortunately, NAION usually is unilateral. But, there are some cases of bilateral disease, which imparts a devastating visual outcome. In the case of bilateral disease, it will usually affect one eye at a time.
There are reported cases of bilateral simultaneous NAION. However, a national study in 1995 found optic nerve fenestration surgery—which once held promise in NAION—to have profound complications with little benefit, so the study was halted.5 Currently, the National Institutes of Health has begun a study utilizing neuro-protective agents in the treatment of NAION.
Arteritic Anterior Ischemic Optic Neuropathy
Patients with AION in association with GCA are classified as having AAION. GCA is the leading cause of AAION; less common causes of arteritis are polyarteritis nodosa, lupus erythematosus and herpes zoster.4 GCA represents a vasculitis of the large- and medium-sized arteries of the head and neck. It has a special predilection to affect the posterior ciliary artery, which is the main blood supply to the optic nerve head. Artery involvement below the aortic arch is rare. Microscopically, there is inflammation of the arterial wall, which is patchy or segmental. This infiltration of giant cells causes a closure of the artery lumen by disruption of the internal elastic lamina.
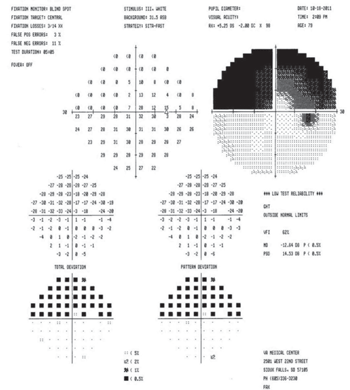
3. There will be a unilateral visual field defect with a classic
altitudinal hemianopsia.
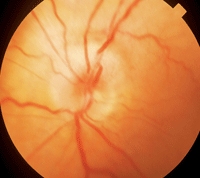
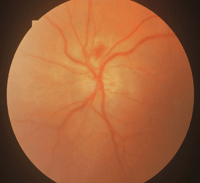
GCA sometimes presents with classic symptoms, which makes the diagnosis more straightforward. But often, however, the diagnosis is difficult to confirm until the patient has lost vision in one eye. The ocular presentation of AAION in GCA is very similar to NAION. Usually the depth of vision loss is greater and the optic nerve shows a chalky (
figure 5) or yellow, waxy appearance (
figure 6).
Splinter hemorrhages of the optic nerve are common. There will be a visual field defect in just one eye that is altitudinal or inferior nasal. Pupillary testing will show an APD of the involved eye. After the optic nerve edema clears, the patient will develop optic atrophy either in a segmental or diffuse pattern. As the optic atrophy progresses, some of these patients will develop optic nerve cupping that looks just like glaucoma—except that the optic nerve pallor is greater than the cupping.6 Most of these patients end up with very poor vision in the affected eye.
GCA patients can have episodes of amaurosis fugax prior to the acute loss of vision. Other less common ocular findings in GCA include retinal artery occlusion, cilio-retinal artery occlusion and sixth nerve palsy. The patient may or may not have a palpable, hard or tender temporal artery. Sohan Singh Hayreh, M.D., M.S., Ph.D., D.Sc., has reported a delay or loss of filling of the short posterior ciliary arteries with fluorescein angiography.4 The more critical issue will be an index of suspicion from constitutional symptoms, including temporal pain, pain with chewing (jaw claudication), scalp tenderness, headache, neck pain, malaise, weight loss, migratory arthropathy and nocturnal sweating. These patients usually will have only some of these symptoms, and different symptoms will occur at different times in their disease. GCA patients have an increased risk of stroke, cardiovascular disease and aortic aneurysm.
Patients who are diagnosed with polymyalgia rheumatica (PMR) have a 15% to 30% probability of developing GCA.6 Some studies report that PMR occurs in about 50% of patients with GCA.7,8 Both conditions exhibit similarities, and many experts believe them to be manifestations of the same disease; others believe GCA and PMR represent two different diseases, supported by human leukocyte antigen typing.9 There is a clinical sub-group in GCA that will not have constitutional symptoms. These patients usually will not present for medical evaluation until they have lost vision in one eye, and must be evaluated for GCA before they potentially lose vision in both eyes from AAION.
Other Related Conditions
• Foster-Kennedy syndrome. If symptoms are ignored or the diagnosis for GCA is overlooked, the patient may later present with pseudo-Foster-Kennedy syndrome.10 This has the clinical appearance of optic atrophy in one eye and papillitis in the fellow eye in the absence of cranial mass. In this presentation, neuroimaging may be needed to rule out Foster-Kennedy syndrome. This presentation also can occur in the infrequent case of bilateral NAION.
In Foster-Kennedy syndrome, optic atrophy is observed in one eye and disc edema in the fellow eye from an intracranial mass lesion.11 The mass most typically is located in the basal frontal area or a sphenoid wing meningioma. Two simple clinical facts can help clarify the diagnosis. First, the visual field defects relating to ION usually will present with an altitudinal defect in the more recent eye. Second, there will be a clear separation of the visual episodes between the two eyes in ION. In Foster-Kennedy syndrome, the vision loss is—to some degree—bilateral with asymmetry. The visual field defects usually are bizarre or respect the vertical midline.
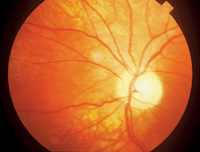
4. NAION often progresses to optic atrophy with a poor visual outcome.
• PION. Like the anterior portion of the optic nerve, the posterior portion of the optic nerve can have an acute infarction—but it occurs less frequently. Just as GCA can induce AION, it less frequently can cause PION. Patients with PION present with a sudden loss of vision in one or both eyes.
Typically, the patient with exhibit a central visual field defect alone or in conjunction with other types of visual field defects in the affected eye or eyes. There is an APD, and initially, the optic nerve looks normal, but then turns pale over four to six weeks. There are three forms of PION: arteritic (APION), non-arteritic (NAPION) and surgical.4
APION is managed just like AAION; however, there is no effective treatment for NAPION. Surgical PION occurs most often in patients who have had non-ocular surgery of longer duration, or have had significant blood loss. PION also occurs in patients who have had a major hemorrhagic event from trauma or a ruptured blood vessel, producing a shock-induced neuropathy. There are no effective treatments for surgical PION, only preventative measures.
| Treatment with Multiple Sclerosis |
| Prior to the Controlled High-Risk Subjects
Avonex MS Prevention Study (CHAMPS), the recommended treatment was
derived from ONTT—an initially high dosage of intravenous
methylprednisolone followed by oral prednisone.1 The CHAMPS study showed
significant treatment benefits with the use of Avonex (interferon
beta-1a, Biogen Inc.) following initial IV methylprednisolone.17 Avonex
is delivered intramuscularly on a weekly basis.
This treatment protocol showed a 57% reduction in the mean number of new T2 lesions, a 67% reduction in the number of gadolinium-enhancing lesions and a 91% reduction in T2 lesion volume. The Avonex-treated group showed a 44% reduction in the probability of developing future multiple sclerosis events in a three-year period.18 The CHAMPS results were so convincing that it was stopped early to make this treatment option available in MS therapy.17 Avonex’s mechanism of action is unknown at this time. It has been proposed that Avonex works by altering the imbalance in the immune system in those with demyelinating disease. In addition to optic neuritis, young patients with internuclear ophthalmopledgia or sixth nerve palsy who show risk of MS should also be considered for Avonex therapy.17 On Sept. 22, 2010, the FDA approved Gilenya (fingolimod, Novartis) capsules for the oral treatment of MS. It is used in MS to reduce relapses and delay disability progression in patients with relapsing forms of MS. It falls within a new class of drugs used in MS treatment that block some blood cells in the lymph nodes, reducing their migration in the brain and spinal cord, which can reduce the severity of MS. Ophthalmic consultation has been advised with this medication due to reported cases of decreased vision from macular edema. Macular edema typically presents three months after the initiation of treatment with Gilenya. |
Some patients with NAPION can retain functional vision, but patients with APION or surgical PION usually are left with very poor vision in the affected eye(s). PION is a diagnosis by exclusion, and other causes must first be eliminated in the differential diagnosis.12
Among many others, differential diagnoses might include central nervous system disease from cerebral mass, cerebral vascular accident, cerebral aneurysm, subdural hemorrhage and meningitis. Ocular etiologies also would need to be ruled out including central retinal artery occlusion, central retinal vein occlusion, retinal detachment and other visible causes of potential eye disease determined by a comprehensive ye examination. The diagnosis of surgical PION is usually more straightforward as the patient recovers from surgery with profound vision loss.
GCA Work-Up
All patients with AION will require a GCA work-up for diagnosis, or diagnosis by exclusion in NAION. All patients suspected of having AION should have blood work done. The work up for GCA should include complete blood count with differential and platelets, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). CRP has gained significant respect as a more specific predictor of GCA; however, at this time, both ESR and CRP should be included in the blood work. All patients with an abnormal ESR and/or elevated CRP also should have a temporal artery biopsy. Some patients with GCA can have normal or only mildly elevated ESR in the earlier stage of their disease. If the patient is anemic, the ESR can show a false negative value, which is the reason these patient should also have a complete blood count with the lab work.
It is important to recognize that many other conditions can cause an elevated ESR and CRP, including chronic renal failure, infection, polyarteritis nodosa, PMR, systemic lupus erythematosus, rheumatoid arthritis, rheumatic fever, ulcerative colitis, regional ileitis, cardiac disease, malignant neoplasm and proteinemias.13
If your index of suspicion is high for GCA, a temporal artery biopsy should be performed. If the first temporal artery biopsy is negative, but the index of suspicion remains high for GCA, these patients should be considered for a second temporal artery biopsy on the contralateral side. It is important to note that there is debate about contralateral temporal artery biopsies. The probability of the unilateral biopsy being negative with a positive biopsy on the contralateral side is 1% to 5%, depending on the study.14
The temporal artery biopsy should obtain a minimum 2cm to 2.5cm section of artery. To improve the yield of the biopsy, some advise a specimen of 3cm to 5cm if possible, which will help minimize “skip areas” for inaccurate pathology. Others recommend the patient should have a bilateral temporal artery biopsy with 5cm sections. Some studies have shown a 13% increase in positive biopsy for GCA with simultaneous bilateral temporal artery biopsies.15
In reality, the surgeon performing the biopsy will dictate the protocol based on what seems best for each individual patient and the surgeon’s individual philosophies. Temporal artery biopsy should be performed within two weeks of starting steroid treatment.16 It is important to note that some patients with GCA can have a negative temporal artery biopsy, because the temporal arteries are not always involved in GCA. For this reason, some patients will be managed for GCA even with a negative temporal artery biopsy. This would be based on at-risk clinical findings, including constitutional symptoms, age, abnormal ESR, abnormal CRP and the presentation of an acute AION.
Treatment of GCA and AAION
The treatment of AAION requires systemic steroids. Patients with AION who are highly suspect of GCA should be started on high-dosage oral steroids (60mg to 100mg) immediately, until a decision can be made in the differential diagnosis. Some clinicians prefer IV steroids initially in the treatment of GCA. These patients are hospitalized and treated with 250mg of methylprednisolone every six hours, for a total of 12 doses. After that, they are treated with oral steroids on a tapering schedule.
5, 6. Usually the depth of vision loss in AAION is greater and the optic nerve shows a chalky (left) or yellow, waxy appearance (right).
A delay in high-dose steroid treatment can result in bilateral blindness in a patient with GCA. If the patient has GCA, long-term oral steroids will be required. The oral steroid dosage will be reduced gradually and titrated based on ESR and CRP levels. Oral steroids never should be adjusted based on the patient’s symptoms or a standard timetable—the rate of reduction must be patient specific. The patient’s internist will be the one to best manage this disease in most cases. Rheumatology consultation and management can be very beneficial in these cases of GCA. Certainly, periodic follow-up with the patient’s eye doctor is important.
Investigation of New Testing in GCA
There is a definite need for improved objective testing in GCA. Currently, several imaging techniques are under investigation to aid in the diagnosis of GCA. These tests include magnetic resonance imaging with contrast media of the vessels, duplex ultrasound and positron emission tomography scanning. A more specific, objective test would be very beneficial in evaluating GCA, because current testing can leave questions about how to manage patients who have a negative temporal artery biopsy but remain highly suspect of having GCA. Similarly, current lab tests can fall short of reaching a definitive diagnosis in patients who are suspected of having GCA. Therefore, laboratory testing that is more specific for GCA also would be very beneficial.
This article should help you better understand the diagnosis and management of the unilateral, acute swollen optic nerve. Some of these neuropathies require no treatment, while others require possible treatment but are not considered an ophthalmic emergency. Most importantly, one subtype of acute optic neuropathy is a true ophthalmic emergency requiring immediate treatment to prevent blindness—which in most cases is avoidable with timely diagnosis and treatment.
Optometrists must be prepared to manage patients with acute optic neuropathies. Some patients will require a team approach of different specialists working together for a timely diagnosis and appropriate treatment. When you encounter a case relating to optic nerve diseases in which the diagnosis remains a mystery, cover yourself and the patient with neuroimaging.
Dr. Tassi practices hospital-based optometry at the VA Medical Center in Sioux Falls, S.D. Dr. VanderZee practices hospital-based optometry at the same facility, where he serves as chief of optometry, and also is a fellow of the American Academy of Optometry.
1. Hayreh SS. Ischemic optic neuropathy. Prog Retin Eye Res. 2009 Jan;28(1):34-62.
2. The clinical profile of optic neuritis. Experience of the Optic Nerve Treatment Trial. Optic Neuritis Study Group. Arch Ophthalmol. 1991 Dec;109(12):1673-8.
3. Desai N, Patel MR, Prisant LM, Thomas DA. Nonarteritic anterior ischemic optic neuropathy, J Clin Hypertens (Greenwich). 2005 Feb;(7)2:130-3.
4. Hayreh SS. Management of ischemic optic neuropathies. Indian J Ophthalmol. 2011 Mar-Apr;59(2):123-36.
5. Ischemic Optic Neuropathy Decompression Trial Research Group. Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA. 1995 Feb 22;273(8):625-32.
6. Gonzalez-Gay MA, Garcia-Porrua C, Salvarani C, Hunder GG. Diagnostic approach in a patient presenting with polymyalgia. Clin Exp Rheumatol. 1999 May-Jun;17(3):276-8.
7. Salvarani C, Cantini F, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. Lancet. 2008;372(9634):234-45.
8. Brooks RC, McGee SR. Diagnostic dilemmas in polymyalgia rheumatica. Arch Intern Med. 1997 Jan 27;157(2):162-8.
9. Cantini F, Niccoli L, Storri L, et al. Are polymyalgia rheumatica and giant cell arteritis the same disease? Semin Arthritis Rheum. 2004 Apr;33(5);294-301.
10. Shatz NJ, Smith JL. Non-tumor causes of the Foster Kennedy syndrome. J Neurosurg. 1967 Jul;27(1):37-44.
11. Watnick RL, Trobe JD. Bilateral optic nerve compression as a mechanism for the Foster Kennedy syndrome. Ophthalmology. 1989 Dec;96(12):1793-8.
12. Hayreh SS. Posterior ischemic optic neuropathy. Ophthalmologica.1981;182(1):29-41.
13. Louisiana State University Health Shreveport. Outpatient management manual: laboratory tests. Available at:
www.sh.LSUHSC.edu/fanmed/outpatientmanual/content.html (accessed March 24, 2012).
14. Boyev LR, Miller NR, Green WR. Efficacy of unilateral versus bilateral temporal artery biopsies for the diagnosis of giant cell arteritis. Am J Ophthalmol. 1999 Aug;128(2):211-5.
15. Breuer GS, Nesher G, Nesher R. Rate of discordant findings in bilateral temporal artery biopsy to diagnose giant cell arteritis. J Rheumatol. 2009 Apr;36(4):794-6.
16. Achkar AA, Lie JT, Hunder GG, et al. How does previous corticosteroid treatment affect the biopsy findings in giant cell (temporal) arteritis? Ann Intern Med 1994 Jun;120(12):987-92.
17. CHAMPS Study Group. Interferon beta-1a for optic neuritis patients at high risk for multiple sclerosis. Am J Ophthalmol. 2001 Oct;132(4):463-71.
18. Jacobs LD, Beck RW, Simon JH. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med. 2000 Sep 28;343(13):898-904.
19. Miller NR, Newman NJ, Biousse V, Kerrison JB. Walsh & Hoyt’s Clinical Neuro-Ophthalmology: The Essentials. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2007.

