Every optometrist has stories of patients whose systemic disease(s) was only diagnosed after the onset of ocular symptoms and signs. Systemic processes can impact virtually every ocular tissue, as well as the orbit and visual pathway. Early detection, diagnosis and treatment of both the ocular manifestations and the underlying systemic condition can lead to improved visual outcomes and a reduction in severe health complications and morbidity.
While optometrists would be unlikely to identify kidney disease through an eye exam alone, when we do encounter a patient with a known history of kidney disease or one of the oculorenal syndromes, we need to be vigilant for the ocular manifestations.
The eye and the kidney share developmental, physiological and pathogenic pathways. Both the glomerulus and choroid have vascular networks that are similar in structure. The renin–angiotensin–aldosterone system (RAAS) is found in both the kidney and in various ocular tissues. This system is an important regulator of blood volume and systemic vascular resistance. Renin, angiotensin II and aldosterone act to elevate arterial pressure in response to decreased renal blood pressure, decreased salt delivery to the distal convoluted tubule and/or beta-agonism. Through these mechanisms, the body can elevate the blood pressure in a prolonged manner.6
In addition to the systemic RAAS, tissue-specific regulatory systems have been described in various organs, including the eye. These local regulatory systems, such as the one present in the retinal vascular endothelium, are responsible for physiologic changes. A local RAAS and its components have been detected in many structures of the human eye, from a possible role in aqueous humor dynamics and intraocular pressure to retinal vascular implications in hypertension and diabetes.7
Here, we will give you a clinical look at the eye in renal disease.
 |
|
Click image to enlarge. |
The Oculorenal Syndromes
Interestingly, the period of organogenesis for both the eyes and the kidneys spans the fourth to sixth weeks of gestation.8 Therefore, any deficits in embryogenesis during this time frame can cause anatomic and functional abnormalities in the two organs. A variety of human congenital oculorenal syndromes affecting both the eye and the kidney have been described.9
WAGR syndrome affects several body systems and is named for its main features: Wilms tumor, aniridia, genitourinary anomalies and intellectual disability (formerly referred to as mental retardation). Most people with WAGR syndrome have aniridia, typically the first noticeable sign.10
People with WAGR syndrome have a 45% to 60% chance of developing Wilms tumor, a rare form of kidney cancer that is most often diagnosed in children.10 Patients with Wilms tumor are at an increased risk for developing ocular disorders, including aniridia and, less frequently, optic nerve hypoplasia resulting from inactivation of the aniridia gene Pax6.10,11 Other ocular signs may also develop, such as cataracts, glaucoma and nystagmus.
The association of ocular coloboma with urinary anomalies may evoke a renal-coloboma or papillorenal syndrome, with coloboma involving the optic disc and adjacent retina. These patients have renal hypoplasia, with or without renal failure.12
Von Hippel-Lindau (VHL) syndrome is transmitted as an autosomal-dominant trait with variable penetrance. Its clinical manifestations include cerebellar and retinal hemangioblastomas, pancreatic cysts, renal cell carcinoma and pheochromocytoma.13 The VHL gene is on chromosome 3 (3p25-26) and functions normally as a tumor suppressor by inhibiting transcription elongation.14 Hemangioblastoma is a round, red tumor of the retina with a pair of feeding vessels showing an increase in diameter and tortuosity. It is benign.
Sturge-Weber syndrome (SWS) is a dermato-oculo-neural syndrome involving cutaneous facial nevus flammeus in the area of the first and/or second division of the trigeminal nerve, ipsilateral glaucoma, ipsilateral diffuse cavernous hemangioma of the choroid and ipsilateral leptomeningeal hemangioma.15 The ocular component of SWS may also manifest vascular malformations of the conjunctiva, episclera, choroid and retina.
 |
|
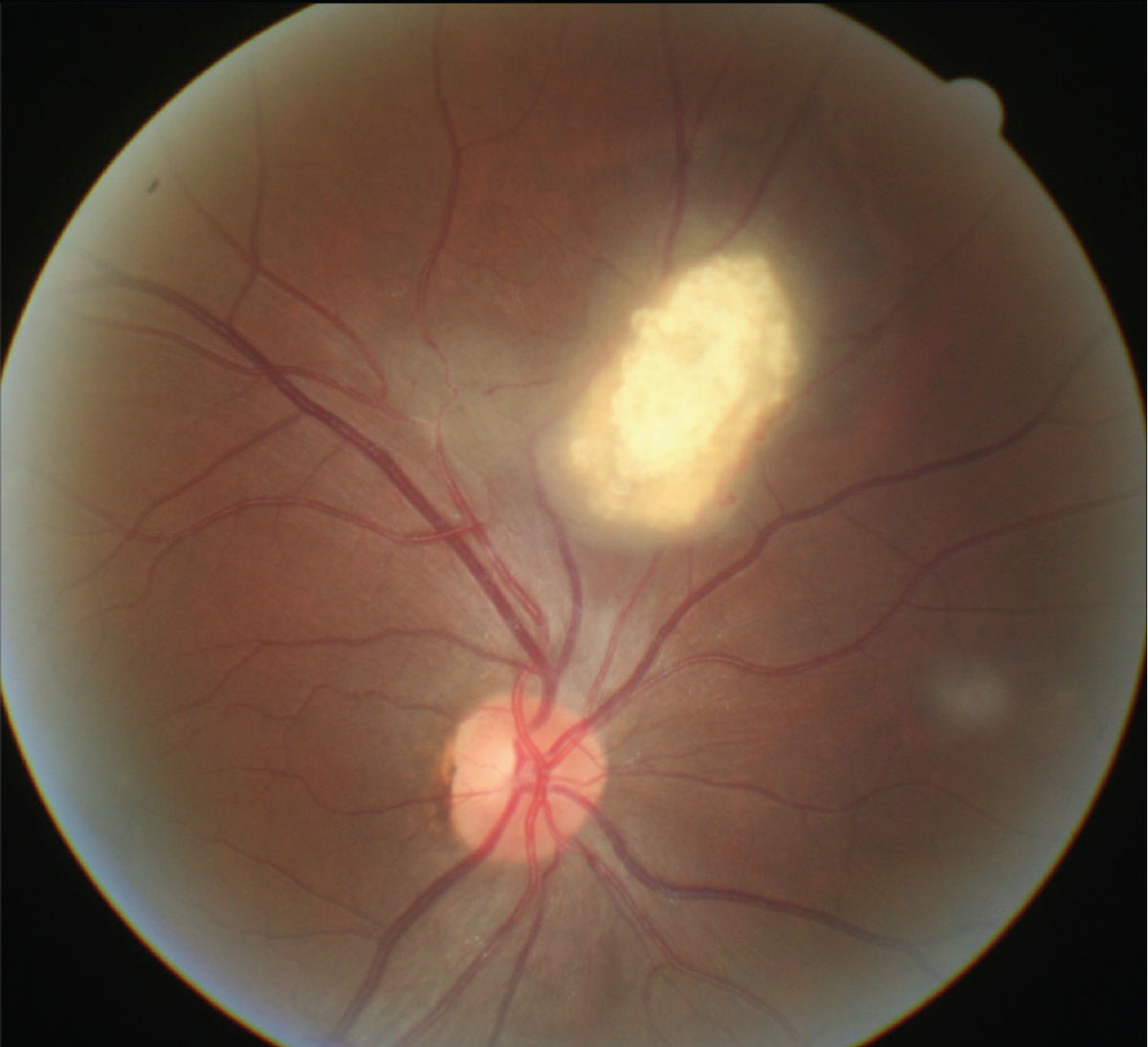
Fig. 2. The most suggestive ocular finding in TSC is retinal astrocytic hamartoma. Click image to enlarge. |
Tuberous sclerosis complex (TSC) is an autosomal-dominant disease with variable penetrance. It is a multisytem disorder characterized by the formation of angiomyolipomas or tubers affecting the brain, skin (called adenoma sebaceum), kidneys, eyes and heart.8 The most suggestive ocular finding in TSC is retinal astrocytic hamartoma, found in 50% to 85% of patients (Figure 2).8,16 Two different genetic loci have been identified in TSC: one on chromosome 9 (TSC1) and another on chromosome 16p (TSC2) that is immediately adjacent to the gene for the most common form of autosomal dominant polycystic kidney disease.8,16
Bardet-Biedl syndrome (BBS) is a genetically heterogeneous disorder with the primary features of obesity, pigmentary retinopathy, polydactyly, renal malformations, intellectual disability and hypogenitalism. BBS is considered an autosomal recessive disorder that increases patient risk for developing diabetes, hypertension and congenital heart disease. Ocular abnormalities include rod-cone dystrophy, strabismus and cataract.8,17
Alport syndrome (AS). The classic phenotype as described by Alport is nephritis, often progressing to renal failure, and sensorineural hearing loss affecting both sexes in successive generations.21 The ocular manifestations (present in 15% to 30%) of AS mainly involve the lens. Bilateral anterior lenticonus is the most cited specific abnormality.8,19
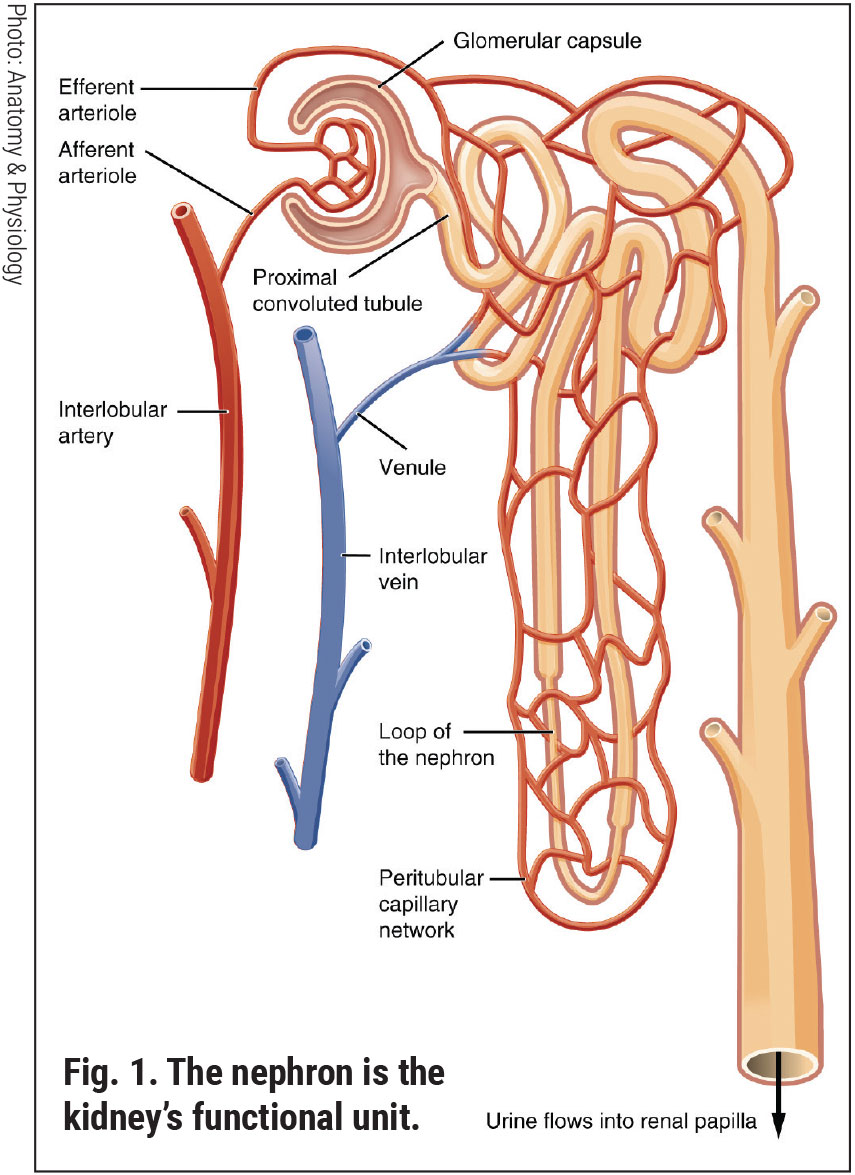
The Kidneys: Functional Anatomy and PathophysiologyEach of our two kidneys is about the size of a fist. Contained within each kidney are roughly a million nephrons. Comprised of a coiled tubule and an extensive network of capillaries, the nephron is the functional unit of the kidney (Figure 1).1 Each nephron houses a special filter, or glomerulus, which filters our blood, removing toxins and excess water. Its thin walls allow smaller molecules, wastes and fluids to pass into the tubule, which ultimately is excreted as urine. Larger molecules, such as proteins and blood cells, do not get filtered and remain in the blood vessel. The renal tubule returns needed substances back to the blood stream. The two kidneys together filter about 200 liters of fluid every 24 hours.2 Renal health is central to maintaining correct balances and effective functioning of all the cells of the body. As cells break down, they produce acids. The extracellular matrix requires a stable composition of salts (such as sodium and potassium) and acidity. The foods we eat can either increase or lower the amount of acid in the body. Our kidneys balance the body’s pH by either removing or adjusting the amounts of acid and buffering agents.1,2 The kidney is capable of detecting and responding to low levels of oxygen (hypoxia) through increased production of erythropoietin.3 One adrenal gland sits on top of each kidney. These endocrine glands produce hormones that regulate metabolism, immune function, blood pressure and response to stress.1,2 Vitamin D has many roles in the body, including modulation of cell growth, neuromuscular function and glucose metabolism. Replacement of vitamin D in deficient populations by supplementation could reduce premature morbidity and mortality rates.4 The kidneys are essential in helping the body use vitamin D. Evidence of a new, non-classical role for vitamin D has emerged to show regulation of the renin-angiotensin system and the nuclear factor (NF) κB pathway.4 Chronically damaged kidneys have a weaker ability to convert vitamin D into its active form. Such patients may benefit from greater effort toward improving vitamin D intake (Table 1). Most renal diseases attack the nephrons, leaving the kidney unable to remove waste. Causes of renal disease include genetic mutations, injuries and certain medicines. People are at a higher risk of kidney disease if they have hypertension, diabetes, heart disease or a first-degree relative with renal issues.5 In chronic kidney disease (CKD), the nephrons are slowly and progressively damaged over many years. Some common diseases of the kidney include cysts, stones, infections and cancers.
|
The Eye in Chronic Renal Disease
Chronic kidney disease (CKD) is characterized by a gradual loss of kidney function over time. CKD may be caused by uncontrolled diabetes. Hypertension causes CKD and CKD causes hypertension. Persistent proteinuria (protein in the urine) means CKD is present. High-risk groups for CKD include those with diabetes, hypertension and family history of kidney failure. African-Americans, Hispanics, Pacific Islanders, American Indians and seniors are at increased risk.
Glomerulonephritis is a family of diseases that cause inflammation and damage to the nephron. These disorders are the third most common type of kidney disease, after hypertension and diabetes. Inherited conditions, such as polycystic kidney disease, cause large cysts to form in the kidneys and damage of the surrounding tissue.
Lupus and other diseases that affect the immune system are associated with renal issues. Obstructions may be caused by problems like kidney stones, tumors or an enlarged prostate gland. Repeated urinary infections may also result in CKD.20,21
Chronic kidney disease has long been tied to eye disorders, including retinopathy (diabetic and hypertensive), glaucoma and cataract. Researchers recently found a high prevalence of visual impairment and major eye diseases in patients with chronic kidney disease (CKD)—and a strong association between the two. The investigators discovered that the prevalence of visual impairment and major eye diseases was two- to seven-fold higher in participants with CKD. They noted that CKD was associated with visual impairment, ocular disease and retinopathy, including diabetic retinopathy.22
In the Chronic Renal Insufficiency Cohort (CRIC) study group, investigators examined fundus photographs of 1,936 patients with varying stages of CKD.23 They found that 45% had retinal microvascular abnormalities that required ophthalmic follow-up, while 3% had serious retinal vascular lesions that required urgent treatment.
Further results from CRIC indicated that a glomerular filtration rate (GFR) of <30mL/min per 1.73m2 was associated with a three-times greater risk of developing retinopathy compared to patients with normal GFR.24,25 Retinal vascular abnormalities may indicate the concurrent presence of macrovascular damage, such as cardiovascular disease (CVD), even after adjustment for renal dysfunction and traditional CVD risk factors.25
There was a strong association between the severity of retinopathy and kidney function. This association remained after adjustment for risk factors for CKD, suggesting that the retinal vascular changes reflect renal disease.24,25 When we see rapid progression of retinal microvascular abnormalities on a dilated fundus examination, this suggests that renal function may be compromised and the patient needs referral to a nephrologist for assessment and management of the diabetic nephropathy. What is going on in the retinal ecosystem is likely also happening in the glomerulus. The assessment of retinal morphology using fundus imaging and OCT/OCT-angiography may prove valuable in future studies of CVD in those with CKD.
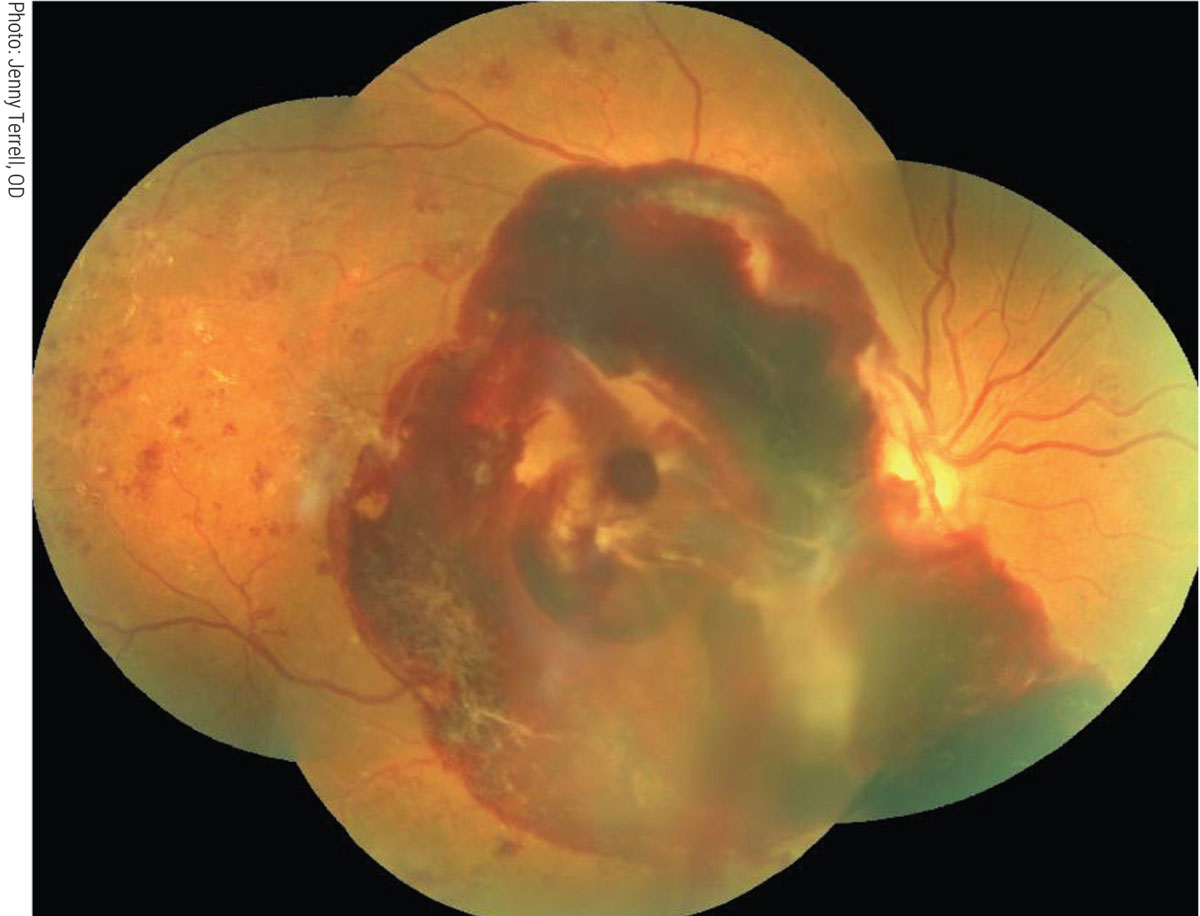
Severe vision loss due to proliferative diabetic retinopathy (PDR) or diabetic macular edema (DME) is about five times more common in diabetic patients with a glomerulopathy compared with non-albuminuric patients (Figure 3).26 Hypertensive retinopathy can be particularly severe in renal failure, and accelerated hypertension may result in bilateral optic disc edema. Aggressive treatment for diabetic kidney disease might help prevent the progression of DR. Alternatively, patients treated with maintenance hemodialysis may experience systemic hypotension, a common side effect of ultrafiltration.26,27 Bansal reported on two dialyzed patients who developed severe longstanding hypotension that suffered bilateral non-arteritic anterior ischemic optic neuropathy.27
Diabetic retinopathy patients with CKD require closer surveillance and more frequent or aggressive retinal treatment than those without. These treatments include intravitreal anti-VEGF agents, laser photocoagulation and, when necessary, surgical retina management.
 |
|
Fig. 3. Severe vision loss due to PDR more common in diabetic patients with chronic kidney disease. Click image to enlarge. |
One of the tests that may be of great value in patients with CKD and retinal vasculopathy is intravenous fluorescein angiography. Although generally considered safe for patients receiving dialysis, one manufacturer of fluorescein suggests using half the normal dose in dialyzed patients.28
In some types of transplantation (such as lung and liver), post-transplant malignancies tend to occur in the transplanted organ. In kidney transplantation, this does not appear to be the case. Patients who have undergone kidney transplantation are at a higher risk for squamous cell carcinoma (SCC), owing to postoperative immune suppression. The risk of developing SCC is about 100 times higher after a transplant. SCC and other post-kidney transplant malignancies may affect the various tissues of the eye, orbit and adnexa.29
Renal cell carcinoma metastasizing to the eye and orbit are rare but should be considered in differential diagnosis of mass lesions. In patients presenting with atypical orbital or ocular masses, the possibility of renal cell carcinoma metastasis should be examined, especially if there is a history of previous renal disorder.30
Diagnosis of Renal Disease
The National Kidney Foundation (NKF) and the National Kidney Disease Education Program (NKDEP) recommend that people at high risk be screened for kidney disease. The NKF recommends that everyone with diabetes between the ages of 12 and 70 be screened at least once a year. In addition to blood pressure and glycemic testing (HbA1c), serology and urinalysis may be performed to detect kidney disease. Specific tests of renal anatomic integrity and function include:
• Glomerular filtration rate (GFR): A common blood test that checks for CKD. The GFR indicates how well the kidneys are filtering. There are several methods to test GFR. Most commonly, the rate is estimated by measuring another substance. Many estimated glomerular filtration rate (eGFR) tests use a formula based on the levels of creatinine, a waste product produced by the body’s muscles, in the blood. The eGFR result should be displayed in milliliters per minute per body surface area. In adults, a normal eGFR number is more than 90 milliliters per minute per 1.73 square meters of body surface area (mL/min/1/.73mm2).
• Blood urea nitrogen (BUN) and creatinine testing: Frequently ordered together as part of a basic or comprehensive metabolic panel (BMP or CMP), these are groups of tests done to evaluate the health of major organs.
– BUN: This measures the amount of urea in a sample of blood. Urea is a waste product that forms as part of the body’s natural process of breaking down proteins. It is also referred to as urea nitrogen and is filtered out of the blood by the kidneys. When BUN levels are too high, it can be an indication that the kidneys are not functioning properly. In general, around 6mg/dL to 24mg/dL (2.1mmol/L to 8.5mmol/L) is considered normal.
– Creatinine (blood and urine): This test measures the amount of creatinine in the blood and/or urine. Reference range varies by sex and age. An increased creatinine level in the blood may mean that the kidneys are not working as they should.
• Urine albumin and albumin-creatinine ratio: This is used for screening and diagnosis of kidney disease. They can also help track the progression of disease and how well the kidneys respond to treatment.
– The protein albumin can pass into the urine if the glomerular capillaries suffer damage. This is similar to the pathogenesis of retinal exudate formation in diabetic retinopathy.
– For an albumin-to-creatinine ratio test, the result will generally be listed in milligrams of albumin per gram of creatinine (mg/g). For a 24-hour urine sample, the total grams of albumin in the full day’s sample will be shown (g/day or g/24 hours).
– Abnormally high values on this pair of tests are an indication of renal microvascular disease.
• Imaging
– Ultrasonography
– CT, MRI or CT urography
• Renal biopsy
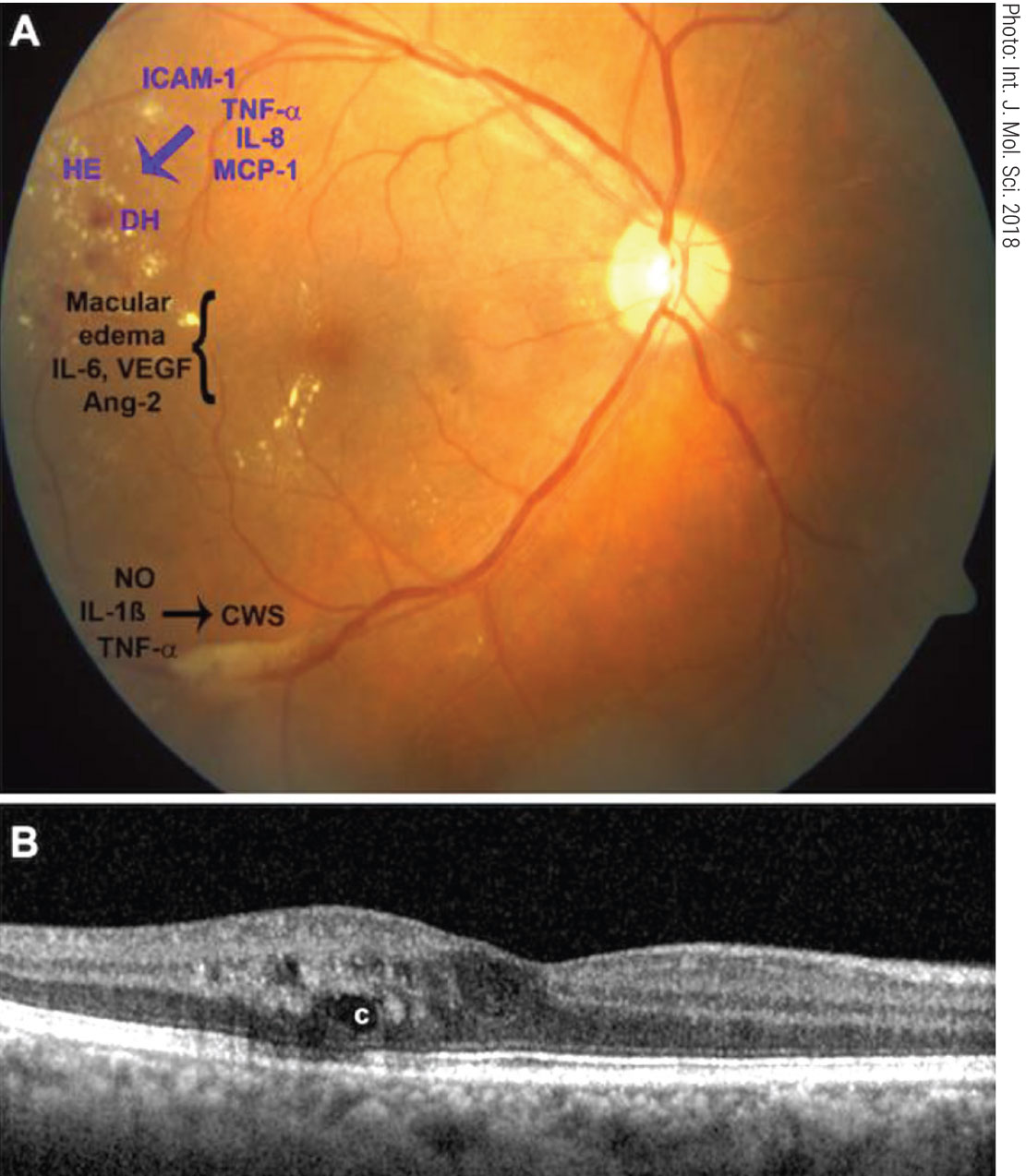 |
|
Fig. 4. Interferon retinopathy clinical appearance and pro-inflammatory cytokines (A). Exudates and cystic edema on OCT (B). Click image to enlarge. |
Management of Renal Disease
Depending on the underlying cause, some types of renal disease can be treated. Management usually consists of measures to control signs and symptoms, reduce complications and slow progression. Medications to lower the blood pressure—such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers—also help preserve kidney function.20 Care should be taken, however, as these pharmacotherapies can initially change electrolyte levels and decrease kidney function.
A diuretic agent and low-salt diet may be recommended, especially if there is fluid retention. Statins may be prescribed, as people with CKD often experience dyslipidemia.20 If the patient becomes anemic, the nephrologist may recommend supplements of the hormone erythropoietin, sometimes with added iron.3,20 These will aid in production of more red blood cells, which may relieve the fatigue and weakness associated with anemia.
Treatment for end-stage kidney disease requires dialysis or a kidney transplant. Transplanted kidneys can come from deceased or living donors. Without such intervention, patients in complete or near-complete kidney failure have a life expectancy of only a few months.
The OD’s Role
Optometrists can greatly contribute to the ocular and overall wellness of patients living with renal disease by following some essential tenets of care.
1. An annual comprehensive eye examination should be performed in patients with any renal condition, especially those with chronic or end-stage kidney disease. Examine the patient for signs of the oculorenal syndromes and retinal vascular disease.
2. Retinal vascular findings may reflect not only cardiovascular problems but also renal disease. The severity of retinal vasculopathy appears to vary with kidney function. Similar risk factors may be affecting the progression of both retinal and chronic renal disease.2
3. Chronic kidney disease impacts diabetic and hypertensive retinopathies. Aggressive treatment for diabetic kidney disease might prevent or slow the progression of diabetic and hypertensive retinopathies.20,21 Facilitate a consultation with nephrology in these cases.
4. Intensive therapy of proteinuria may protect the eye. One study reported that remission of microalbuminuria with intensive therapy appeared to be a significant protective factor for the development of PDR and DME.2
5. Patients with chronic renal disease can lose vision. Researchers recently found a high prevalence of visual impairment and major eye diseases in patients with CKD and a strong association between the two.22 Best-corrected vision of 20/40 or below was two to seven times more common in such patients. These findings highlight the importance of comprehensive eye examinations in CKD patients. There is a potential common pathogenesis underlying these conditions.25,3
6. Drugs being taken for renal disease have adverse ocular side effects. Diuretics have a strong link with progression of glaucoma. Calcium channel blockers have also been linked to progression of glaucoma. However, the ACE inhibitors may be neuroprotective as well as renoprotective.31
Interferon is used to treat various conditions including renal cell carcinoma. Interferon can lead to retinal damage appearing two to 12 weeks after the start of treatment (Figure 4). The retinopathy usually presents as cotton wool spots and retinal hemorrhages in the posterior pole.32,33
Most patients with early to moderate interferon retinopathy are asymptomatic. With that said, functional vision loss may occur and can be irreversible in some patients even after discontinuation of therapy. Branch retinal artery and vein occlusion, central retinal vein occlusion, cystoid macular edema and optic disc edema have all been associated with interferon therapy.33 Patients with diabetes or hypertension or who are taking interferon are more likely to experience interferon retinopathy and should be closely monitored.
7. Drugs being taken for ocular diseases may require renal evaluation and monitoring. Most drugs and their active metabolites are eliminated through the kidneys. Therefore, all medications must be considered systemically potent in patients with poor renal function. Before prescribing a systemic agent, clinicians should question the patient about a history of renal disease. Systemic nonsteroidal anti-inflammatory drugs, steroids and antiviral agents are just some of the agents used in eye care that warrant further history and assessment of drug clearance ability.
Antiviral drugs cause renal failure through a variety of mechanisms. Direct renal tubular toxicity has been described with a number of medications with unique effects on epithelial cells of the kidney. Dosage adjustment according to renal function is indicated for many drugs.
The Bottom Line
To summarize, there exists significant evidence of a close association between renal and ocular wellness—and illness. Recognizing and understanding the links may ultimately lead to the development of new diagnostic and management strategies for both types of diseases.
Dr. Pizzimenti is a professor at the Rosenberg School of Optometry, University of the Incarnate Word. He is also a fellow of both the American Academy of Optometry and the Optometric Retina Society. He has no financial interests to disclose.
Dr. Pelino is an assistant professor at the Pennsylvania College of Optometry, Salus University. He is also a fellow of the American Academy of Optometry and a member of the Optometric Retina Society. He has no financial interests to disclose.
1. Lote CJ. Principles of renal physiology, 5th edition. Springer. 2012. 2. Ogobuiro I, Tuma F. Physiology, renal. StatPearls Publishing. August 29, 2020. [Epub ahead of print]. 3. Jelkmann W. Regulation of erythropoietin production. J. Physiology. 2011 Mar 15; 589(Pt 6):1251-8. 4. Williams S, Malatesta K, Norris K. Vitamin D and chronic kidney disease. Ethn Dis. 2009;19(4 Suppl 5):S5-11. 5. Kazancioğlu R. Risk factors for chronic kidney disease: an update. Kidney Int Suppl (2011). 2013;3(4):368-371. 6. Fountain JH, Lappin SL. Physiology, renin angiotensin system. StatPearls Publishing. 2021. [Epub ahead of print]. 7. Toshihide Kurihara, Yoko Ozawa, Susumu Ishida, et al. “Renin-angiotensin system hyperactivation can induce inflammation and retinal neural dysfunction.” Int J of Inflammation. 2012;2012:1-14. 8. Bodaghi B, Massamba N, Izzedine H. The eye: a window on kidney diseases. Clin Kidney J. 2014;7(4):337-8. 9. Pohl M, Bhatnagar V, Mendoza SA, et al. Toward an etiological classification of developmental disorders of the kidney and upper urinary tract. Kidney Int. 2002;61:10–19. 10. Fischbach BV, Trout KL, Lewis J, et al. WAGR syndrome: a clinical review of 54 cases. Pediatrics. 2005 Oct;116(4):984-988. 11. Dahl E, Koseki H, Balling R. Pax genes and organogenesis. Bioessays. 1997;19(9):755-765. 12. Schimmenti LA, Cunliffe HE, McNoe LA, et al. Further delineation of renal-coloboma syndrome in patients with extreme variability of phenotype and identical PAX2 mutations. Am J Hum Genet. 1997;60(4):869-878. 13. Chauveau D, Duvic C, Chretien Y, et al. Renal involvement in von Hippel-Lindau disease. Kidney Int. 1996;50(3):944–51. 14. Duan DR, Pause A, Burgess WH, et al. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science. 1995;269(5229):1402-6. 15. Sullivan TJ, Clarke MP, Morin JD. The ocular manifestations of the Sturge-Weber syndrome. J Pediatr Ophthalmol Strabismus. 1992;29(6):349-356. 16. Hyman MH, Whittemore VH. National Institutes of Health consensus conference: Tuberous sclerosis complex. Arch Neurol. 2000;57(5):662-5. 17. Beales PL, Reid HA, Griffiths MH, Maher ER, Flinter FA, Woolf AS: Renal cancer and malformations in relatives of patients with Bardet-Biedl syndrome. Nephrol Dial Transplant. 2000;15:1977-1985. 18. Alport, AC. Hereditary familial congenital hemorrhagic nephritis. Brit Med J. 1927;1:504-6. 19. Jacobs M, Jeffrey B, Kriss A, et al. Ophthalmologic assessment of young patients with Alport syndrome. Ophthalmology. 1992;99:39-1044. 20. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1294-1304. 21. Hsieh YT, Hsieh MC. Time-sequential correlations between diabetic kidney disease and diabetic retinopathy in type 2 diabetes—an eight-year prospective cohort study. Acta Ophthalmologica. 292;99(1):e1-6. 22. Zhu Z, Liao H, Wang W, et al. Visual impairment and major eye diseases in chronic kidney disease: the National Health and Nutrition Examination Survey 2005 to 2008. Am J Ophthalmol. 2020;213:24-33. 23. Grunwald JE, Alexander J, Ying GS, et al. Retinopathy and chronic kidney disease in the Chronic Renal Insufficiency Cohort (CRIC) study. Arch Ophthalmol. 2012;130(9):1136-1144. 24. Important findings from CRIC. Chronic Renal Insufficiency Cohort Study. www.cristudy.org/Chronic-Kidney-Disease/Chronic-Renal-Insufficiency-Cohort-Study/findings-from-cric. Accessed August 5, 2021. 25. Grunwald JE, Pistilli M, Ying GS, et al. Association between progression of retinopathy and concurrent progression of kidney disease: findings from the chronic renal insufficiency cohort (CRIC) study. JAMA Ophthalmol. 2019;137(7):767-74. 26. Bodaghi B, Massamba N, Izzedine H. The eye: a window on kidney diseases. Clin Kidney J. 2014;7(4):337-8. 27. Bansal S, Ansons A, Vishwanath M. Hypotension induced blindness in haemodialysis patients. Clin Kidney J. 2014;7:387-90. 28. AK-Fluor. Package insert. Akorn. 2021. 29. Witmanowski H, Lewandowska M, Szychta P, Sporny S, Rykała J. The development of squamous cell carcinoma in a patient after kidney transplantation: a case report. Postepy Dermatol Alergol. 2013;1:65-71. 30. Shome D, Honavar SG, Gupta P, Vemuganti GK, Reddy PV. Metastasis to the eye and orbit from renal cell carcinoma--a report of three cases and review of literature. Surv Ophthalmol. 2007;52(2):213-23. 31. Park SJ, Byun S, Park JY, et al. Primary open-angle glaucoma and increased risk of chronic kidney disease. Glaucoma. October 17, 2019. [Epub ahead of print]. 32. Hejny C, Sternberg P, Lawson DH, et al. Retinopathy associated with high-dose interferon alfa-2b therapy. Am J Ophthalmol. 2001;131:782-7. 33. Rubio JE Jr, Charles S. Interferon-associated combined branch retinal artery and central retinal vein obstruction. Retina. 2003;23:546-8. |