The advent of and improvements in ultra-widefield imaging (UWFI) technology now provide clinicians an excellent view of the posterior segment without dilation. On the surface, the technology offers many benefits. For the patient, it can decrease in-office wait time and eliminate any side effects of dilation. For the clinician, it can provide an opportunity to use a patient’s own retinal image to discuss findings—thus improving patient care with visual education. But limitations to these instruments still exist, and most clinicians continue to question their place in clinical practice. This article takes a closer look at the standards of care for when dilated fundus examination (DFE) is indicated and where this new technology fits in.
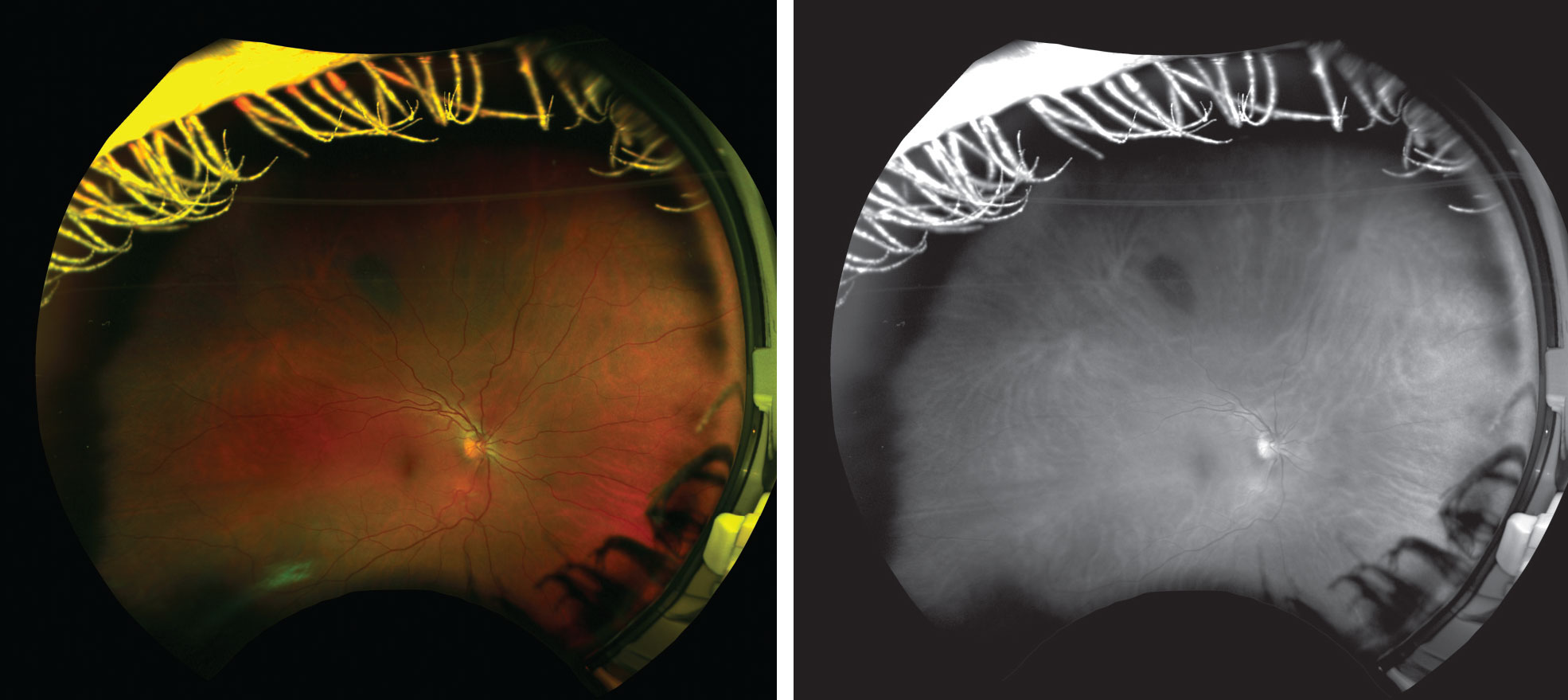 |
| Figs. 1 and 2. This patient has a longstanding choroidal nevus in the superior midperiphery that appears very light in color during funduscopy and could be easily overlooked if the biomicroscope is too bright and bleaches out the faint nevus. Even in the color UWFI image (left), it is somewhat difficult to appreciate; however, the green-free image (right) provides a clear view. |
Set Your Standards Straight
A dilated fundus examination is considered the standard of care.1 In fact, an optometrist could be legally liable if a UWFI image fails to identify disease that could be reasonably proven was present at the time of the examination but was missed because of lack of dilation. DFEs remain the best method to maximally and stereoscopically visualize the posterior segment compared with UWFI alone from a legal, ethical and clinical standpoint. And when a UWFI instrument does image a suspicious retinal lesion, dilation is still the standard to further evaluate and accurately diagnose the finding.2
Despite this, some question the support for routinely dilating healthy, asymptomatic patients. Studies show that routine DFE has a low yield for discovery of serious ocular events and may be ineffective in altering the course and outcome of incidental findings, even at 10-year intervals in asymptomatic patients.3 Others have found that dilated exams yield clinical findings in approximately 5% of asymptomatic, low-risk patients and few of these findings are beyond the view of a direct ophthalmoscope or undilated indirect exam and even fewer were in need of treatment or intervention.4 Despite the paucity of support in the literature, considering the ocular disease we can identify in those 5% of patients, it is ethically and legally our responsibility to perform regular DFEs, even on asymptomatic patients. UWFI without dilation should never be offered as a universal option to all patients, as it is below clinical standard of care.
Given the current standard, the choice to image rather than dilate remains a medical decision best made on a case-by-case basis. To make the right decision, clinicians must understand the evidence-based studies, the pros and cons of UWFI devices and dilation and the pitfalls of imaging alone.
Restricted AccessIn our practice, patients are only eligible for UWFI screening in lieu of dilation under several conditions:
Other considerations include the patient’s refractive error, family history and current list of medications. The UWFI screening option is only offered to patients on a case-by-case basis by the doctors and is never discussed as an option by our staff. We do not have a universal option for patients to choose dilation vs. UWFI themselves. |
Consider the Evidence
UWFI performance compared with DFE varies based on the disease studied. Clinicians should carefully consider the evidence of commonly encountered posterior segment symptoms and diseases before recommending UWFI to the patient.
Diabetes. Given that nearly 86% of individuals with type 1 diabetes and 40% of those with type 2 diabetes have some form of clinically evident diabetic retinopathy (DR), the American Optometric Association’s (AOA) Evidence Based Clinical Practical Guidelines and the American Academy of Ophthalmology’s (AAO) Preferred Practice Pattern, recommend individuals with diabetes receive at least annual dilated eye examinations.5-7 More frequent exams may be needed depending on the presence of DR, and the AOA outlines specific recommendations based on the severity.5
Several studies have demonstrated good agreement between Optomap UWFI (Optos) and dilated funduscopy of grading DR by doctors of varying levels of expertise.8 Of the discrepancies noted, there were minimal to no instances where the difference in grading would have significantly or adversely affected patient outcomes. In fact, data shows a tendency for clinical grading to be less severe than image grading, which could be a potential source of clinical risk if it delays treatment, as the disease severity changes the recommended follow-up schedule.8
Generally, studies conclude that although results seem promising for UWFI as a telemedicine screening tool in diabetes, a larger study size is required before it can be considered the standard of care.8-10
Primary open-angle glaucoma. The AOA and the AAO recommend an examination of the optic nerve that requires stereoscopic visualization with adequate magnification.11,12 To achieve stereopsis, the pupils must be dilated and the patient examined with a 78D or 90D lens. Evaluation of the optic nerve also includes ruling out other potential causes of optic atrophy or subtle abnormalities that might result in visual field loss similar to that caused by glaucoma.11,12 Therefore, patients with glaucoma should be dilated on a regular basis to best assess definitive optic nerve head changes with stereopsis, which is still considered the standard for monitoring glaucoma.13
Currently, no reliable studies compare dilation with UWFI for grading either cup-to-disc ratio or the rate of glaucoma diagnosis and management. However, several studies show good agreement and high reproducibility in the evaluation of vertical cup-to-disc ratio compared with stereoscopic optic disc imaging, suggesting UWFI may be helpful for glaucoma diagnosis in situations in which standard color digital stereoscopy is not available.14
In some instances, certain UWFI features that allow easier assessment of the retinal nerve fiber layer (RNFL) may help improve glaucoma diagnosis and management, including red-free imaging and fundus autofluorescence (FAF). Although UWFI is not specifically designed to quantifiably measure RNFL loss, subtle RNFL defects seen using red-free images may indicate early glaucomatous damage before the development of glaucomatous optic nerve cupping.
Visible RNFL loss on red-free imaging should be further investigated as a potential indicator of glaucoma or other optic neuropathy as you would with other clinically identifiable risk factors. Red-free serial imaging can also be used to monitor for RNFL wedge defect progression over time.15 Additionally, FAF can detect and monitor the extent of peripapillary atrophy, although not enough evidence exists to correlate hypo-FAF in peripapillary atrophy to functional glaucomatous damage.16
Age-related macular degeneration (AMD). Both the AOA and the AAO recommend stereoscopic biomicroscopic examination of the macula.17,18 Even conservative recommendations include comprehensive examinations with dilation every one to two years after the age of 65 to catch the subtle early signs of macular degeneration.19
Patients diagnosed with AMD require dilation at appropriate intervals, depending on disease severity, to detect the earliest signs of choroidal neovascularization.
No studies show that color image UWFI has apparent benefits over dilated exams for the diagnosis or management of macular degeneration. While ongoing studies are evaluating the ability to phenotype the retinal periphery with UWFI to monitor peripheral pathologic changes in AMD, these peripheral grading criteria are difficult to assimilate into clinical practice.20,21
Posterior vitreous detachment and peripheral vitreoretinal disease. According to the AOA, binocular indirect ophthalmoscopy with pupillary dilation is generally necessary to diagnose a peripheral retinal break or detachment with scleral depression, if indicated. The AAO’s Preferred Practice Patterns specifically states “wide-field color photography can detect some peripheral retinal breaks but does not replace careful ophthalmoscopy” for peripheral vitreoretinal disease.22,23
Several studies compare dilation with UWFI modalities for non-traumatic retinal breaks, and most agree that UWFI is a useful adjunct for documentation, but its ability to detect the break, especially in the inferior and superior periphery, is low to moderate compared with DFE.24 One study shows that for retinal lesions posterior to the equator, sensitivity of detection was 74%; however, for anterior lesions it was only 45%.25 Furthermore, occasional instrument artifacts can result in a false positive diagnosis of retinal detachment, choroidal lesions, vascular inflammation or retinal elevation, which causes undue stress to the patient. Thus, dilation remains the standard for detecting retinal tears in new symptomatic patients for peripheral retinal break.
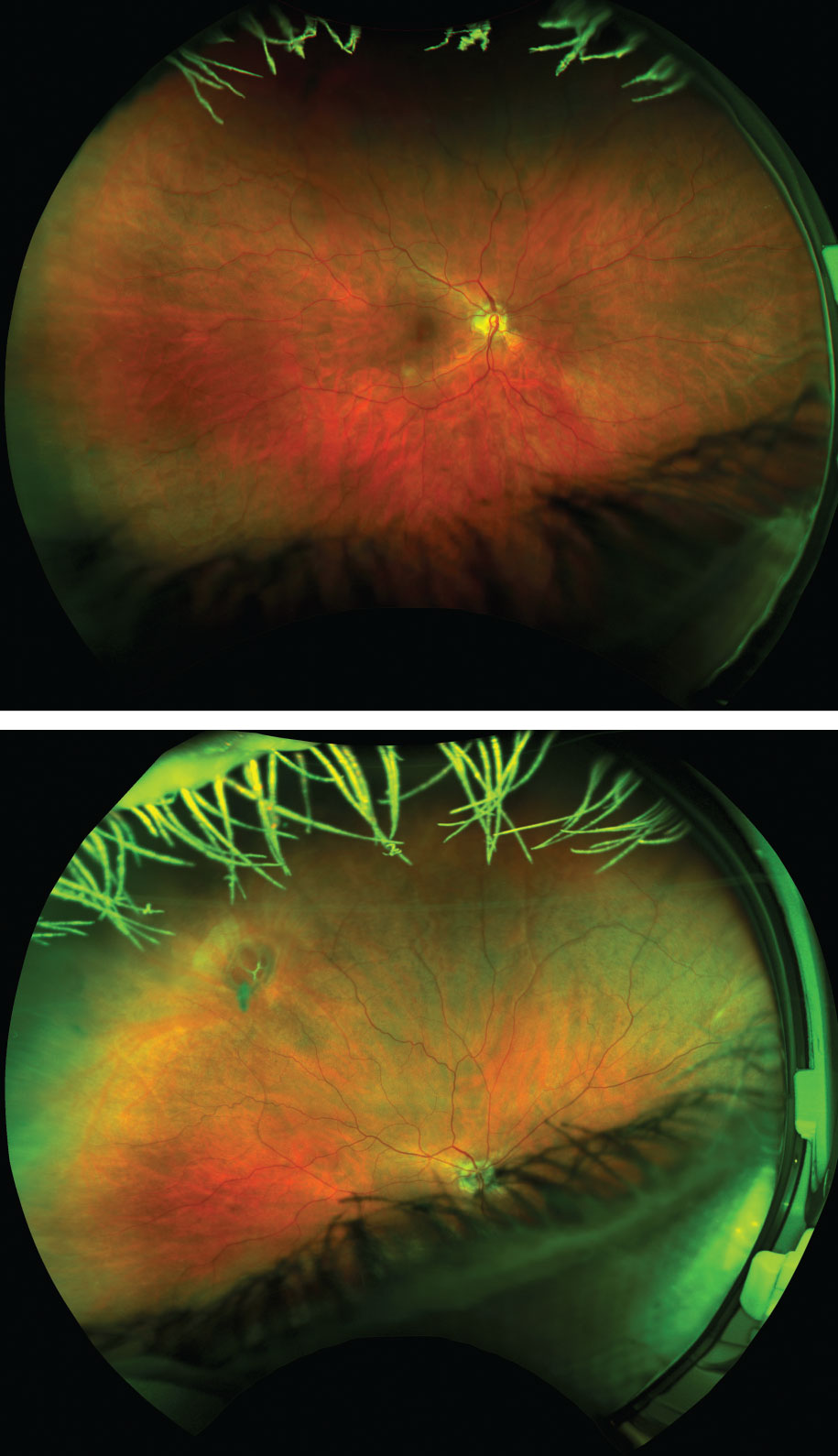 |
| Figs. 3 and 4. Despite no entering symptoms, a DFE revealed a large operculum with four large retinal holes with surrounding subretinal fluid. Optomap imaging was recommended to photodocument the finding, and the initial photograph (top) was wide and reliable with the patient fixating straight as per routine protocol. After instructing the patient to look in the direction of known pathology, the holes were easily detected by the instrument (bottom). If this patient had not been dilated and UWFI were used to assess retinal health alone in primary gaze, her image would have been read as unremarkable. Instead, a thorough DFE enabled prompt diagnosis and same-day laser retinopexy. |
The Asymptomatic, Low-risk Patient
Few would argue that dilation is the preferred method of evaluation in the presence of known ocular disease or symptoms. However, significant contradictory evidence exists on the subject of annual dilation for asymptomatic, low-risk patients.
No strong evidence defines the optimal frequency of eye exams of patients younger than 65 with no ocular symptoms or signs. In fact, some evidence suggests the diagnostic yield of DFEs in asymptomatic patients is not high, particularly in younger age groups.26,27 Both the AAO and AOA acknowledge the lack of published research to support or refute the use of routine pharmacologic dilation in asymptomatic, low risk patients.1,19
The majority of studies comparing dilation with UWFI in asymptomatic, low-risk patients agree with a sensitivity and specificity of approximately 75%, which draws the conclusion that UWFI is a potential alternative to dilation. In addition, because of the automated red-free and green-free images offered with most devices, UWFI can sometimes be more sensitive for subtle findings such as small peripheral hemorrhages or microaneurysms and faint choroidal nevi.28,29
The Pros of UWFI
Several benefits of using UWFI to examine posterior segment health exist. In addition to patient convenience and the elimination of the side effects of dilation, it also impresses patients. Some may even seek out offices that are known to offer UWFI to avoid dilation if possible, which makes this technology quite profitable, as it is typically an additional out-of-pocket charge.
It also provides the doctor several advantages. It creates a permanent visual record clinicians can use to educate patients about their ocular health and any findings suspicious for diabetes, hypertension and other diseases. It can also document serial imaging over years to prove change over time and track progression. In addition, some pathology is actually more noticeable in imaging than dilated examinations when using the red-free and green-free images. For many conditions, seeing a wide view of the retina provides context to more accurately diagnose lesions (Figures 1 and 2).30
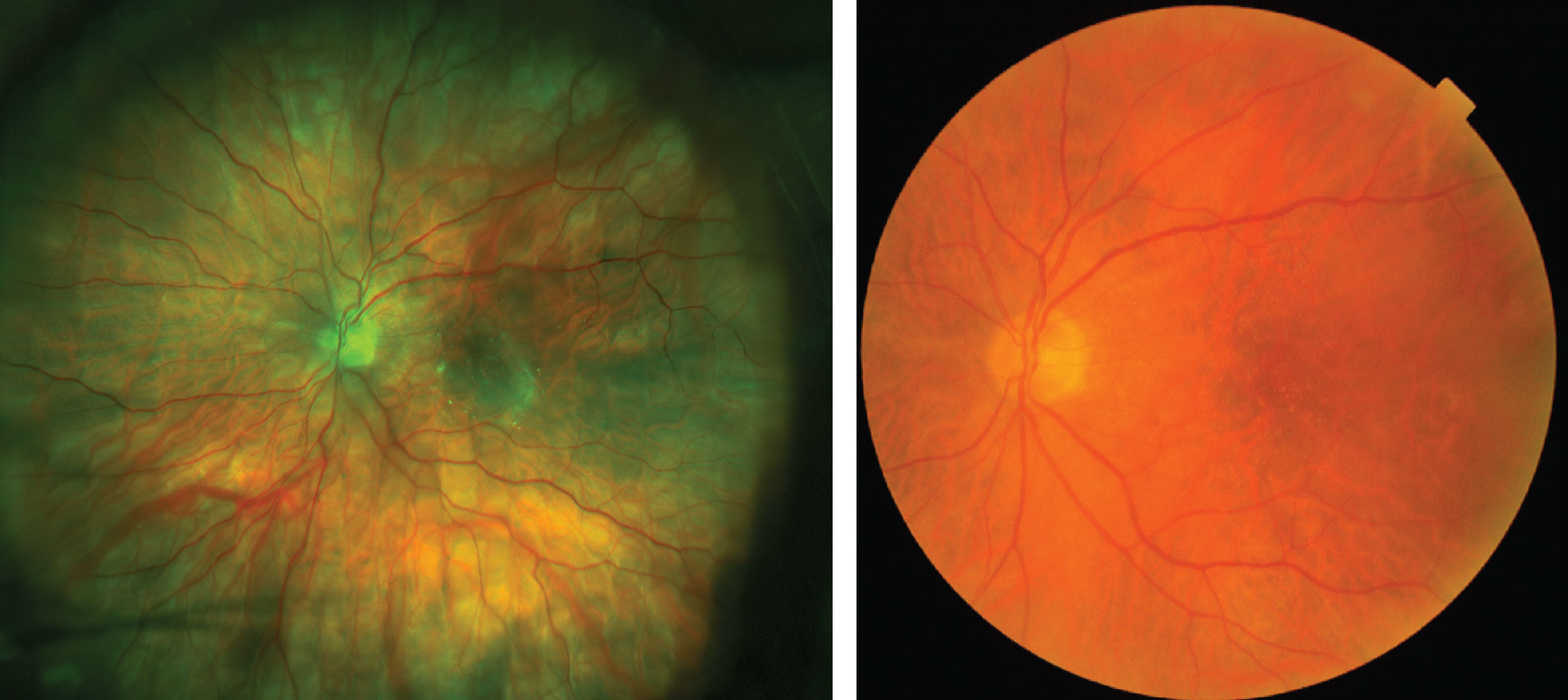 |
| Figs. 5 and 6. This patient has significant fine drusen throughout the posterior pole that was difficult to image using UWFI, left. If UWFI were the only modality used to monitor this patient’s retinal health, it would likely be interpreted as normal. Instead, this patient’s fundus photograph, right, clearly shows well defined, hard, small drusen scattered in the posterior pole, leading to further testing and a diagnosis of early AMD. |
The Limitations of UWFI
The most significant limitation of these devices is the inability to image the entire retina. Approximately 18% of the retina cannot be imaged, even through a dilated pupil with current technology.30 Furthermore, this value is based on best-case scenario imaging. In a clinic setting, not every patient images well due to poor patient attention, dry eye, ocular media obscurations, lid ptosis or small pupils and/or dark choroid causing a dark image. Within most studies of UWFI, approximately 10% of images taken were of insufficient quality to interpret. Sometimes the anterior retinal pathology found during funduscopy cannot be imaged with UWFI, even with a fully dilated pupil (Figures 3 and 4).
Furthermore, because the UWFI image is an artificial composite of red and green light sources and uses an elliptical mirror to capture the widefield image, most UWFI devices often do not capture fine macular detail to the degree that a DFE or a traditional dilated macula photograph does. In some cases, artifacts in the macular region can preclude accurate diagnosis of more subtle abnormalities, particularly in the case of fine drusen that is indicative of early macular degeneration (Figures 5 and 6).31
The Combo Approach
One of the best clinical uses of UWFI is as an adjunct to DFE. Research shows an Optomap-assisted fundus examination can improve pathology detection and help the clinician efficiently target an area of the retina during funduscopy in need of further investigation. Having a widefield image prior to performing the DFE improves the rate and accuracy of posterior segment disease diagnosis. One study showed a 30% increase in retinal lesion discovery compared with traditional DFE alone.32
UWFI can be particularly useful in diagnosing and managing peripheral as well as posterior retinal pathology when used with other widefield imaging modalities such as red-free and green-free imaging, fundus autofluorescence, fluorescein angiography and indocyanine green angiography.
While standards have yet to formally change, UWFI continues to affect the landscape of diagnosing and monitoring retinal pathology. It can be an invaluable adjunct to the traditional DFE for improving the rate of pathology detection, and it can help to capture peripheral lesions for medical photodocumentation. In cases of known disease, UWFI cannot replace a dilated fundus examination altogether. But in asymptomatic, low-risk patients, it may be a beneficial screening modality. It should never take away from our ability to care for each patient as an individual prior to advising a universal management protocol in lieu of what is still considered the standard of care.
Dr. Legge is in private practice in Wyomissing, PA.
1. AOA Evidence-based Optometry Committee. Evidence-Based Clinical Practice Guideline. Comprehensive Adult Eye and Vision Examination. American Optometric Association. 2015. 2. Bailey RN, Heitman E, eds. An Optometrist’s Guide to Clinical Ethics. American Optometric Association, 2000. www.aoa.org/documents/optometrists/book.pdf. Accessed September 12, 2018. 3. Varner P. How frequently should asymptomatic patients be dilated? J Optom. 2014;7(1):57-61. 4. Pooack AL, Brodie SE. Diagnostic yield of the routine dilated fundus examination. Ophthalmology. 1998;105(2):382-6. 5. AOA Evidence-based Optometry Committee. Evidence-based Clinical Practice Guideline: Eye Care of the Patient with Diabetes Mellitus. 2014. 6. American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern Guidelines. Diabetic Retinopathy. American Academy of Ophthalmology. 2017. 7. Hazin R, Barazi MK, Summerfield M. Challenges to establishing nationwide diabetic retinopathy screening programs. Curr Opin Ophthalmol. 2011;22(3):174-9. 8. Purbrick R, Izadi S, Gupta A, Chong NV. Comparison of Optomap ultrawide-field imaging versus slit-lamp biomicroscopy for assessment of diabetic retinopathy in a real-life clinic. Clin Ophthalmol. 2014;8:1413-7. 9. Sallam A, Scanlon PH, Stratton IM, et al. Agreement and reasons for disagreement between photographic and hospital biomicroscopy grading of diabetic retinopathy. Diabet Med. 2011; 28(6):741-6. 10. Neubauer AS, Kernt M, Haritoglou C, et al. Nonmydriatic screening for diabetic retinopathy by ultra-widefield scanning laser ophthalmoscopy (Optomap). Graefes Arch Clin Exp Ophthalmol. 2008;246(2):229-35. 11. Fingeret M, Mancil GL, Baily IL, et al. Optometric Clinical Practice Guideline: Care of the Patient with Open Angle Glaucoma. 2010. www.aoa.org/documents/optometrists/CPG-9.pdf. Accessed September 12, 2018. 12. American Academy of Ophthalmology Glaucoma Panel. Preferred Practice Pattern Guidelines. Primary Open-Angle Glaucoma. American Academy of Ophthalmology. 2015. 13. Thomas R, Loibl K, Parikh R. Evaluation of a glaucoma patient. Idian J Ophthalmol. 2011;59(Suppl1):S43-S52. 14. Quinn N, Azuara-Blanco A, Graham K, et al. Can ultra-wide field retinal imaging replace colour digital stereoscopy for glaucoma detection? Ophthal Epidem. 2017;5(1):63-9. 15. Sherman J, Patel H, Nath S, et al. Correlation between Optos ultra widefield imaging and traditional diagnostic methods in glaucoma. Ophthalmology. 2009;80(6):302-3. 16. Reznicek L, Seidensticker F, Mann T, et al. Correlation between peripapillary retinal nerve fiber layer thickness and fudus autofluorescence in primary open-angle glaucoma. Clin Ophthalmol. 2013;7:1883-8. 17. American Academy of Ophthalmology Glaucoma Panel. Preferred Practice Pattern Guidelines. Age-Related Macular Degeneration. American Academy of Ophthalmology. 2015. 18. AOA Consensus-based Optometry Committee. Consensus-Based Clinical Practice Guideline. Care of the Patient with Age-Related Macular Degeneration. 2004. 19. American Academy of Ophthalmology Preferred Practice Patterns Guidelines. Comprehensive Adult Medical Eye Evaluation. American Academy of Ophthalmology. 2010. 20. Lengyel I, Csutak A, Florea D, et al. A population-based ultra-widefield digital image grading study for age-related macular degeneration-like lesions at the peripheral retina. Ophthalmology. 2015;122(7):1340-7. 21. Writing Committee for the OPTOS PEripheral RetinA (OPERA) study (Ancillary Study of Age-Related Eye Disease Study 2). Peripheral retinal changes associated with age-related macular degeneration in the Age-Related Eye Disease Study 2: Age-Related Eye Disease Study 2 Report Number 12 by the Age-Related Eye Disease Study 2 Optos PEripheral RetinA (OPERA) Study Research Group. Ophthalmology. 2017 Apr;124(4):479-87. 22. AOA Consensus-based Optometry Committee. Consensus-Based Clinical Practice Guideline: Care of the Patient with Retinal Detachment and Peripheral Vitreoretinal Disease. 2004. 23. American Academy of Ophthalmology Preferred Practice Patterns Guidelines. Posterior Vitreous Detachment, Retinal Breaks, and Lattice Degeneration. American Academy of Ophthalmology. 2014. 24. Kornberg DL, Klufas MA, Yannuzzi NA, et al. Clinical utility of ultra-widefield imaging with the Optos Optomap compared with indirect ophthalmoscopy in the setting of non-traumatic rhegmatogenous retinal detachment. Semin Ophthalmol. 2016;31(5):505-12. 25. Mackenzie P, Russell M, Ma PE, et al. Sensitivity and specificity of the Optos Optomap for detecting peripheral retinal lesions. Retina. 2007;27(8):1119-24. 26. Pollack AL, Brodie SE. Diagnostic yield of the routine dilated fundus examination. Ophthalmology. 1998;105:382-6. 27. Batchelder TJ, Fireman B, Friedman GD, et al. The value of routine dilated pupil screening examination. Arch Ophthalmol. 1997;115:1179-84. 28. Nath S, Sherman J, Battaglia M. Is Optos imaging additive of duplicative to a dilated fundus exam? Invest Ophthalmol Vis Sci. 2005;46(13):1554. 29. Nath S, Sherman J, Hossain SM. Comparison of panoramic imaging (Optos P200C) with traditional dilated retinal evaluation. Invest Ophthalmol Vis Sci. 2009;50(13):339. 30. Shoughy S, Arevalo JF, Kozak I. Update on wide- and ultra-widefield retinal imaging. Indian J Ophthalmol. 2015;63(7):575-81. 31. Pandya AN, Friberg TR, Eller AW. Optos non-mydriatic widefield imaging vs. clinical dilated fundus exam for retinal diagnosis and management. Invest Ophthalmol Vis Sci. 2002;43(13):2860. 32. Brown K, Sewell JM, Trempe C, et al. Comparison of image-assisted versus traditional fundus examination. Eye and Brain. 2013;2013(5):1-8. |

