Earn New Surgical SkillsOptometric surgery is increasingly becoming a core responsibility of the profession. Today, 17 states (and counting) allow ODs to perform laser or other minor surgical procedures. Here to offer tips and advice on surgery implementation and technique is the December 2023 issue of Review of Optometry, the magazine's 30th annual surgery report. Inside, experts offer advice on performing SLT, equipping your office for surgery and handling complications of in-office procedures, along with a review of the newest IOLs on the market. Check out the other articles featured in the December issue: |
Selective laser trabeculoplasty (SLT) has been a mainstay of the glaucoma treatment armamentarium for almost two decades, initially as a second-line therapy after maximum drop therapy. However, SLT has gained steam as primary therapy in open-angle glaucoma and ocular hypertension cases more recently thanks to the LiGHT trial among other studies. Frankly, the literature encouraging optometrists and ophthalmologists alike to change their current thinking and use SLT earlier in the course of therapy is overwhelming at this point. Optometrists in 10 states are currently authorized to perform SLT. The following set of questions and answers will help guide the early adopter of SLT to take the performance of their SLT procedure in their practice to the next level.
1. Does SLT work as primary therapy?
The literature is incredibly strong towards SLT use earlier in the course of therapy. Generally, the literature indicates that the practitioner can expect 20% to 35% intraocular pressure (IOP) lowering for patients where SLT is used as primary therapy. An initial study demonstrated a mean IOP reduction of 23.8% at 26 weeks after a single treatment.1 The SLT/Med Study showed the percentage of IOP reduction nine to 12 months after treatment was 26.4% for the SLT group and 27.8% in the medical/prostaglandin arm with the two treatment arms being statistically equivalent.2
Overall success depends on how it is defined, with the LiGHT trial showing 74.2% of patients being drop-free three years after primary SLT treatment.3 The authors wrote that SLT is effective in 80% to 90% of patients with the effect tending to wane with time. SLT has repeatedly been shown to be equivalent to prostaglandins for first-line therapy, with one study concluding SLT should be offered as a first-line treatment for open-angle glaucoma and ocular hypertension, supporting a change in clinical practice.2,3
The six-year LiGHT trial data released in early 2023 showed 69.8% of SLT patients remained at or below target IOP after six years (they were drop-free). This does not necessarily mean one SLT held the IOP below target for that six-year time period. Of those patients that were drop-free, 62.7% needed only one SLT, 30.9% needed two SLTs and 5.9% needed three SLTs to stay below target over the study period. Most significantly, more eyes in the first-line drop arm exhibited disease progression (26.8%) vs. the eyes in the first-line SLT arm that exhibited disease progression (19.6%). Clearly stated, patients were more likely to progress over a six-year period on eye drops compared to SLT, with compliance likely being the main driver behind that.4
Bottom line: SLT works best as first-line therapy, and eyecare providers are encouraged to use SLT earlier in the course of therapy.
2. Does SLT work as secondary therapy?
SLT has also been investigated as an adjunct treatment for patients on concurrent topical therapy as a means of further IOP reduction. One study reported clinical outcomes of 52 primary open-angle glaucoma (POAG) eyes that received adjunct SLT while on topical medical treatment.5 Average IOP reduction from baseline was 24.3% at one year, 27.8% at two years, 24.5% at three years and 29.3% at four years.
Similar to medications, the effect of SLT as adjunctive therapy is likely not as robust as primary therapy, with the average IOP reduction being approximately 10% to 25% depending on the number of medications the patient is on and the baseline IOP prior to the laser.
Ask yourself, “Which glaucoma medication typically lowers IOP the most?” The first eye drop, the second, the third or the fourth? Generally, it is the first eye drop, with clinical experience showing that this first drop lowers IOP approximately 20% to 30%, the second perhaps 15% to 20%, the third eye drop 5% to 15% and the fourth perhaps zero to 10%.
SLT follows those exact same patterns. If a patient is on three glaucoma medications and then SLT is used, the IOP reduction will likely not be 20% to 30% but perhaps closer to 5% to 10% with the added advantage of helping to blunt the diurnal fluctuations of the IOP, which has been proven to be a significant advantage of SLT.
Bottom line: It does work, just temper your expectations on percentage IOP reduction when an SLT is done on a patient who is already on two, three or more glaucoma medications.
 |
|
Fig. 1. A 360º SLT is being performed with the Rapid SLT lens. We are in mirror #5 of treatment (top left mirror position). Click image to enlarge. |
3. Which medications does SLT pair best with?
When used as adjunctive therapy, SLT pairs well with most all glaucoma drugs. A retrospective review found no difference between specific classes of glaucoma medications in regard to SLT success.6 These findings confirm a role for SLT as an adjunct to glaucoma medications, including prostaglandin analogs (PGAs), which have been suggested to possibly impair the effectiveness of SLT by competing for a common pathway to lower IOP.7 The studies that have suggested that perhaps SLT and PGAs do not pair well together have emphasized the similar mechanisms of lowering IOP for both of them (inflammatory effects via the outflow pathways).
Perhaps it is best for each treatment modality to lower IOP via different mechanisms (e.g., decreased production, increased outflow, lowering episcleral venous pressure). For that reason, at least in theory, SLT could possibly pair best with an aqueous suppressant such as a topical beta-blocker or carbonic anhydrase inhibitor. Clinical experience has shown that SLT pairs well with most topical glaucoma medications.
Bottom line: Clinically speaking, SLT will pair with any glaucoma medication. However, do remember when SLT works best (first-line therapy—see question #1). In theory, SLT pairs best with an aqueous suppressant to maximize the different mechanisms to lower the IOP.
4. When should I not do an SLT?
Knowing when and when not to perform a procedure is critical. SLT is a safe procedure, with few contraindications, though some do exist.
The presence of less than 90° to 180° degrees of visible posterior pigmented trabecular meshwork (TM) on gonioscopy (narrow angles) likely is a contraindication for performing SLT in its current form (see sidebar, “Is There a More ‘Direct’ Approach?”). Numerous secondary glaucomas, including inflammatory, neovascular, angle recession and juvenile, are either absolutely or relatively contraindicated due to the potential for worsening the condition (inflammatory glaucoma), needing other therapy (neovascular glaucoma), or likely non-effectiveness of treatment (angle recession and juvenile glaucoma).
The effect of SLT on the corneal endothelium may be transient, and long-term effects are probably negligible in normal corneas. However, in compromised corneas and corneas with pigment deposits on the endothelium, there may be a risk of corneal endothelial compromise, especially after repeated SLT.8 Therefore, it may be wise to limit the number of shots and energy when considering SLT in a patient with a compromised corneal endothelium.
While age was a contraindication for the previous version of laser trabeculoplasty (argon; ALT), studies show it is not a contraindication for SLT.9
One interesting clarification point: it was once thought that failure of one SLT was a contraindication to perform future SLTs. Some of the leading experts across the world now are providing guidance that just because an SLT failed the first time does not mean it cannot be tried a second or third time.10 Non-response in the LiGHT trial had SLT repeated as soon as eight weeks. The authors were willing to give SLT a second try if it did not give a robust IOP lowering the first time. Generally, we will wait approximately three to 12 months before repeating a failed SLT.
Bottom line: Few contraindications exist for SLT, except for narrow angles, certain secondary glaucomas and possibly corneas with compromised endothelium being the main contraindications.
 |
|
Fig. 2. SLT being done in a heavily pigmented angle. Care should be taken in these types of angles to decrease the energy, use fewer laser pulses over 180° or less and monitor for any post-op IOP spikes closely. Click image to enlarge. |
5. Can I predict which patients are going to be most successful with SLT?
While SLT works well for most, it does not work for all, and selecting patients based on factors that will most likely lead to success of the SLT is critical. Several studies have shown that the strongest predictor of SLT success is higher preoperative IOP.6,11-12
Intuitively, the number of medications a patient is on likely affects the percentage of IOP reduction after SLT, with the more preoperative medications likely leading to a lower percentage of IOP reduction, and vice-versa. One study described results after analysis of 111 eyes treated with 360° SLT, finding that the use of three topical IOP-lowering medications was associated with SLT failure.13 Conversely, another described the five-year success rates of SLT and found no significant difference in success rate on the basis of the number of preoperative glaucoma eye drops patients were using.14 There was, however, an increased likelihood of patients requiring a second procedure (SLT or trabeculectomy) during the five-year follow-up period in those who were taking two or more preoperative IOP lowering drops.
Increased angle pigmentation may correlate with SLT efficacy. In one study, patients were subdivided into three groups on the basis of angle pigmentation.15 Mean IOP decreased by 2.06mm Hg, 2.46mm Hg and 4.75mm Hg in subgroups with low, marked and high angle pigmentation, respectively. Conversely, other studies have shown that angle pigmentation is not predictive of SLT success.16
Regarding the mechanism of action of SLT, pigmentation in the angle is an important variable to consider, as SLT selectively targets melanin to achieve the desired biologic/inflammatory effect. However, angles with low pigmentation likely will still show clinically significant IOP-lowering effects provided treatment protocols are adjusted properly (increasing treatment energy).
Multiple other factors have been investigated yet were not found to be significant predictors of success, including age, sex, previous ALT, angle grade, lens status and central corneal thickness.6
Bottom line: The biggest predictive factor for SLT success (defined as robust percent IOP lowering) is pre-laser IOP (the higher the pre-op IOP, the more likely robust IOP lowering). The second biggest predictive factor for success is the number of meds the patient is on (see question #2); however, there are some studies that refute that.
For example, in which patient, A or B, is the SLT likely going to lower the IOP more?
Patient A: IOP = 25mm Hg on the day of the procedure. SLT used first-line therapy (zero meds).
Patient B: IOP = 18mm Hg on the day of the SLT. Patient is on two glaucoma medications.
The answer is Patient A.
6. How long does the effect of SLT last?
SLT treatment efficacy is known to diminish with time. Survival analysis from past studies indicates that the time for 50% of eyes to fail after SLT treatment is approximately two years.5,17 The LIGHT trial data from 2019 showed 74.2% of patients were drop-free three years after primary SLT treatment.3 The six-year LiGHT trial data released in September 2022 showed 69.8% of SLT patients remained at or below target IOP after six years (they were drop-free). This does not necessarily mean one SLT held the IOP below target for that time period. Of those patients that were drop-free, 62.7% needed only one SLT, 30.9% needed two SLTs and 5.9% needed three SLTs to stay below target over the six-year period.4
If you do the math, there is approximately a 44% chance a single SLT will last six years without the need for further treatment based on the 2022 LiGHT data.4
It is recommended that patients be educated that the likely effectiveness of the procedure lasts somewhere between two to six years, with the option available to repeat the SLT when the IOP elevates or progression is shown.
 |
|
Fig. 3. Very rare angle bleed seen during an SLT. Look just below the aiming beam to see the blood. Click image to enlarge. |
Bottom line: Clinically speaking, SLT has been shown to last between two and six years. Some are longer, some are shorter, depending on number of degrees treated, pre-laser IOP, number of meds the patient is on and general patient idiosyncrasies.
In general, length of efficacy can be hard to predict from patient to patient. The LiGHT trial demonstrated that 44% of first-line SLT patients remained below target IOP without any other intervention (drop-free) from a single SLT at the six-year mark.
7. Is SLT repeatable?
Since SLT causes minimal structural damage to the TM, retreatment is a viable option in patients that need further IOP reduction. Although this benefit of SLT was theoretical for many years, the body of evidence now supports the efficacy of repeat SLT.18 It achieves a similar absolute level of IOP control with mean percent IOP reduction following repeat SLT perhaps slightly lower than the initial treatment. This is possibly related to the retreatment being done at an overall lower level of IOP.
For example, an initial SLT was done at a baseline IOP of 24mm Hg with a 30% IOP reduction to achieve a post-SLT treatment IOP of 17mm Hg. The IOP elevated in the years following the initial SLT to 22mm Hg. Repeat SLT achieved a 25% IOP reduction, taking the IOP back to 17mm Hg. The repeat SLT achieved the same IOP endpoint with a slightly lower percentage of IOP reduction.
One study demonstrated that repeat SLT can maintain IOP at or below target IOP in medication-naïve POAG and ocular hypertension eyes requiring retreatment with at least an equivalent duration of effect to the initial laser.19
Overall, repeat SLT appears to be comparable to initial SLT in regard to efficacy, duration of effect and rate of complications.
Bottom line: SLT is very repeatable, should be repeated and perhaps should be repeated more often. On that note…
8. Should SLT be repeated more often? What about annually?
Considering the mechanism of action of how SLT works, via inflammatory and biologic mechanisms with macrophages and other inflammatory cells “cleaning up” the cellular debris in the TM, it does make sense that SLT done more often could result in enhanced outflow. Why not have the inflammatory cells clean out the drain of the eye (the TM) more often?
With that in mind, researchers from Italy studied the effects of low-energy SLT (half the energy, half the laser pulses, done annually) to observe how that treatment protocol compared to the standard SLT protocol (0.8mJ to 1.0mJ, 80 to 120 laser pulses, repeated as needed often two to six years later) and to traditional ALT.
They found 10-year medication-free rates to be:20
- ALT: 22.6%
- Standard SLT: 25.0%
- Low-energy annual SLT: 58.3%
They also found 10-year median times to medication treatment to be:20
- ALT: 2.8 years
- Standard SLT: 3.2 years
- Low-energy annual SLT: 6.2 years
The results clearly pointed towards a 10-year benefit for the low-energy, annual SLT arm.
The ongoing Clarifying the Optimal Application of SLT Therapy (COAST) trial will be a major indicator of whether lower energy annual SLT will play a big role in our treatment protocols going forward.21 Currently, it appears that the published evidence is trending towards low-energy (half the energy, half the shots), annual SLT. The COAST trial, if it has results similar to the Italy study, likely will guide us towards low-energy, annual SLT just the same way that the LiGHT trial guided us towards SLT as a primary treatment option.
Bottom line: The published literature, and early clinical experience, appears to be trending towards doing a lower energy, annual SLT. Half the energy (0.4mJ to 0.5mJ), half the shots (40 to 60 laser pulses), done annually irrespective of IOP, may be the future SLT protocol. While not clinical standard of care at this point, the COAST trial will go a long way towards clarifying that.
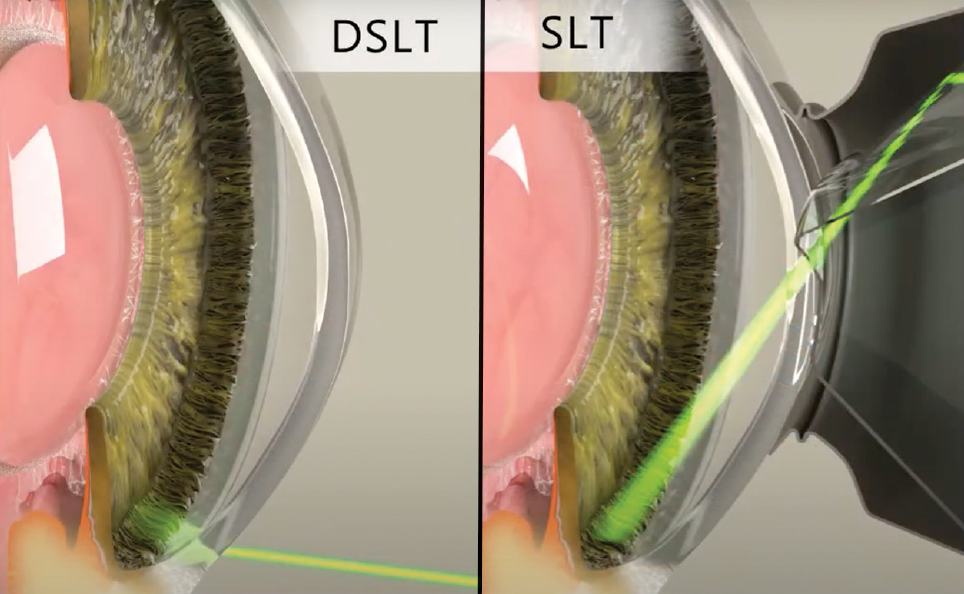
Is There a More “Direct” Approach?Laser trabeculoplasty done in the angle with a gonioscopy laser lens (Latina or Rapid SLT lens) with laser pulses applied directly to the TM is the traditional and current approach. Clinically, it typically takes two to five minutes to perform a 360° SLT depending on experience level, laser lens used and patient factors. As technology has advanced, other approaches have been invented and are currently being studied. An automated device called direct SLT (DSLT) using a transscleral approach is currently approved in Europe and is being studied in the US. What is this new technology and how does it work?
“Automated” means the laser automatically applies the 120 laser pulses without the doctor having to hit the button that many times. Iris tracking allows the laser to align precisely where it wants to fire the laser pulses, which is right on the limbus. With the current DSLT device approved in Europe and awaiting FDA 510(K) clearance in the US (the Belkin Eagle DSLT), treatment is an efficient application of laser energy (Figures 4 and 5). Once the button is pressed, the laser automatically fires 120 laser pulses, as long as the auto-alignment and tracking is providing feedback that the laser is aligned on the limbus. If the patient moves their eye during the treatment, the auto-tracking will detect that and the laser will stop firing.
The “direct” aspect means that the laser pulses are applied directly to the surface of the eye and not into the angle. No laser lens is required to perform the procedure. The laser pulses are applied directly to the limbal area, which anatomically sits just superficial to the angle and TM of the eye. The theory behind the procedure is that the laser pulses are still applied fairly close to the angle and TM, and therefore the inflammatory/biologic reaction that occurs and cleans up the drain in the TM will still occur because the laser pulses are applied close to the area of the drain. Current studies are showing a 15% to 30% IOP reduction, depending on energy level used, for the DSLT.28 Though it hasn't yet reached US shores, it's conceivable that DSLT may revolutionize how SLT therapy is delivered here, as this more automated procedure could encourage greater adoption than the conventional gonio-based technique. |
9. Is 180° or 360° of treatment recommended?
How many angle degrees to treat depends on clinician preference and the type of glaucoma being managed. The early days of SLT generally recommended 180° of treatment, consistent with prior ALT protocols. Due to the mechanism of action, minimal structural damage in the angle, lower side effect profile and repeatability, it has become generally accepted to perform 360° of SLT treatment for POAG, ocular hypertension and NTG.3,22-23
Conversely, some studies indicate that fewer degrees of treatment may be as effective as a full 360° treatment while reducing the incidence and magnitude of postoperative IOP elevation.24-25 Heavier amounts of pigment in the angle can potentially cause an overproduction of inflammation, leading to higher rates of potential adverse events. Therefore, the general consensus is to perform 180° or less in patients with heavy pigmentation in the TM, pigmentary glaucoma or pseudoexfoliative glaucoma.11
Bottom line: 360° (Figure 1) is largely the standard for POAG, ocular hypertension and low-tension glaucoma. 180° or less is recommended for heavily pigmented angles.
10. What are the potential complications to look out for and how do I manage them?
Complications can arise with any treatment, laser or otherwise, and should be dealt with appropriately. SLT is an extremely safe laser procedure, with IOP spike and inflammation being the two most encountered complications. Inflammation is expected due to the mechanism of action of how the SLT laser works.
If too much inflammation, redness or ocular soreness is encountered, it can usually be treated with a topical NSAID or topical steroid for two to four days following the procedure. IOP spikes following laser procedures usually start one to five hours following the laser procedure, and often dissipate within 24 to 72 hours even without treatment.
A transient IOP increase of 5mm Hg or more has been reported in 0% to 28% of eyes.11 More specifically, a study reported transient elevated IOP after SLT of 4.5%, which seems consistent with clinical experience.26 A systematic review found that prophylactic in-office treatment with IOP-lowering medication reduced the incidence of transient IOP elevation.27 Heavily pigmented TMs (Figure 2) may lead to potentially higher rates of IOP spike, so caution should be taken (less shots, lower energy) when performing an SLT on a heavily pigmented angle.
Blurred vision, redness, peripheral anterior synechiae, bleeding/hyphema (Figure 3), ocular soreness, cystoid macular edema, corneal edema and endothelial cell changes have all been rarely reported in the literature and seen clinically.
Bottom line: Complications can arise yet are rare and very treatable, with the most common being an IOP spike.27
Bonus: When can I take the patient off of their glaucoma medications after SLT?
The answer to this very important question is—like many answers in glaucoma—“It depends!” There are two big, broad reasons why SLT is done:
1. To replace a current topical medication, or
2. To prevent the next topical medication (or first medication).
Scenario #1. If a patient is struggling with compliance or adverse effects from glaucoma eye drops such as redness, then the SLT may be done to replace the current medication they are on that is giving them trouble (side effects or compliance). Remember, it takes approximately six weeks to see the full effect of SLT. Depending on the stage of glaucoma, the troublesome medication could be stopped before the SLT is done (if the glaucoma is relatively mild), or six weeks after the SLT is done and has hopefully kicked in (if the glaucoma is more advanced).
Scenario #2. If a patient is progressing (VF or OCT) on one or two medications, or the IOP is not meeting target on one or two meds, then the SLT may be used as an add-on therapy to prevent the patient from going on a second or third drug. In that case, no medication would be stopped, since the SLT is being done to prevent the next med.
Bottom line: It depends on whether you are doing the SLT to replace a current medication or prevent the next (or first) medication.
Takeaways
Optometrists are on the front line of glaucoma treatment and placing patients on their initial glaucoma therapy. There are two clear first-line treatment options: SLT and eye drops. More and more ODs are having the option to perform SLT on their patients, either first- or second-line. Having lasers in practice is a great opportunity, and being confident enough to answer the big questions regarding SLT will help demonstrate to patients your knowledge and expertise.
Dr. Lighthizer is the associate dean, director of continuing education and chief of specialty care clinics at NSUOCO. He is a founding member and immediate past president of the Intrepid Eye Society. Dr. Lighthizer’s full disclosure list can be found here. Dr. Patel is an assistant professor at the Oklahoma College of Optometry (NSUOCO). She completed a Primary Care residency at NSUOCO specializing in ocular disease and advanced procedures. Dr. Patel is a diplomate of the American Board of Optometry and a fellow of the American Academy of Optometry.
1. Latina M, Sibayan S, Shin D, et al. Q-switched 532nm Nd:YAG laser trabeculoplasty (selective laser trabeculoplasty) a multicenter, pilot, clinical study. Ophthalmology. 1998;105(11):2082-90. 2. Katz LJ, Steinmann WC, Kabir A, et al; SLT/Med Study Group. Selective laser trabeculoplasty vs. medical therapy as initial treatment of glaucoma: a prospective, randomized trial. J Glaucoma. 2012;21(7):460-8. 3. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty vs. eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicenter randomied controlled trial. Lancet. 2019;393(10180):1505-16. 4. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Laser in Glaucoma and Ocular Hypertension (LiGHT) Trial: six-year results of primary selective laser trabeculoplasty vs. eye drops for the treatment of glaucoma and ocular hypertension. Ophthalmology. 2023;130(2):139-51 5. Weinand FS, Althen F. Long-term clinical results of selective laser trabeculoplasty in the treatment of primary open-angle glaucoma. Eur J Ophthalmol. 2006;16(1):100-4. 6. Martow E, Hutnik CM, Mao A. SLT and adjunctive medical therapy: a prediction rule analysis. J Glaucoma. 2011;20(4):266-70. 7. Alvarado J, Iguchi R, et al. From the bedside to the bench and back again: predicting and improving the outcomes of SLT glaucoma therapy. Trans Am Ophthalmol Soc. 2009;107: 167-81. 8. Ong K, Ong L, Franzco L. Corneal endothelial changes after selective laser trabeculoplasty. Clin Exp Ophthalmol. 2013;41(6):537-40. 9. Liu D, Chen D, Tan Q, et al. Outcomes of selective laser trabeculoplasty in young patients with primary open angle glaucoma and ocular hypertension. J Ophthalmol. 2020;2020:5742832. 10. Garg A, Vickerstaff V, Nathwani N, et al. Efficacy of repeat selective laser trabeculoplasty in medication-naive open-angle glaucoma and ocular hypertension during the LiGHT Trial. Ophthalmology. 2020;127(4):467-76. 11. Kennedy J, SooHoo J, Kahook M, Seibold L. Selective laser trabeculoplasty: an update. Asia Pac J Ophthalmol (Phila). 2016;5(1):63-9. 12. Hodge WG, Damji KF, Rock W, et al. Baseline IOP predicts selective laser trabeculoplasty success at one-year post-treatment: results from a randomized clinical trial. Br J Ophthalmol. 2005;89(9):1157-60. 13. Lee JW, Liu CC, Chan JC, Lai JSM. Predictors of success in selective laser trabeculoplasty for Chinese open-angle glaucoma. J Glaucoma. 2014;23(5):321-5. 14. Woo DM, Healey PR, Graham SL, Goldberg I. Intraocular pressure-lowering medications and long-term outcomes of selective laser trabeculoplasty. Clin Exp Ophthalmol. 2015;43(4):320-7. 15. Wasyluk JT, Piekarniak-Wozniak A, Grabska-Liberek I. The hypotensive effect of selective laser trabeculoplasty depending on iridocorneal angle pigmentation in primary open angle glaucoma patients. Arch Med Sci. 2014;10(2):306-8. 16. Alon S. Selective laser trabeculoplasty: a clinical review. J Current Glau Prac. 2013;7(2):58-65. 17. Bovell AM, Damji KF, Hodge WG, et al. Long term effects on the lowering of intraocular pressure: selective laser or argon laser trabeculoplasty? Can J Ophthalmol. 2011;46(5):408-13. 18. Guo Y, Ioannidou A, Jute P. Selective laser trabeculoplasty: a review of repeatability. Ann Eye Sci. 2019;4:20. 19. Garg A, Vickerstaff V, Nathwani N, et al. Efficacy of repeat selective laser trabeculoplasty in medication-naive open-angle glaucoma and ocular hypertension during the LiGHT Trial. Ophthalmology 2020;127:467-76. 20. Gandolfi S. Low power selective laser trabeculoplasty (SLT) repeated yearly as primary treatment in open angle glaucoma(s): long term comparison with conventional SLT and ALT. ARVO 2018 annual meeting. 21. Realini T, Gazzard G, Latina M, Kass M. Low-energy selective laser trabeculoplasty repeated annually: rationale for the COAST Trial. J Glaucoma. 2021;30(7):545-51. 22. Prasad N, Murthy S, Dagianis JJ, Latina MA. A comparison of the intervisit intraocular pressure fluctuation after 180 and 360 degrees of selective laser trabeculoplasty (SLT) as a primary therapy in primary open angle glaucoma and ocular hypertension. J Glaucoma. 2009;18(2):157-60. 23. Shibata M, Sugiyama T, Ishida O, et al. Clinical results of selective laser trabeculoplasty in open-angle glaucoma in Japanese eyes: comparison of 180 degree with 360 degree SLT. J Glaucoma. 2012;21(1):17-21. 24. Woo DM, Healey PR, Graham SL, Goldberg I. Intraocular pressure-lowering medications and long-term outcomes of selective laser trabeculoplasty. Clin Experiment Ophthalmol. 2015;43(4):320-7. 25. McAlinden C. Selective laser trabeculoplasty (SLT) vs. other treatment modalities for glaucoma: systematic review. Eye (Lond). 2014;28(3):249-58. 26. Song J. Complications of selective laser trabeculoplasty: a review. Clin Ophthalmol. 2016;10:137-43. 27. Wong MO, Lee JW, Choy BN, et al. Systematic review and meta-analysis on the efficacy of selective laser trabeculoplasty in open-angle glaucoma. Surv Ophthalmol. 2015;60(1):36-50. 28. Goldenfeld M, Belkin M, Dobkin-Bekman M, et al. Automated direct selective laser trabeculoplasty: first prospective clinical trial. Transl Vis Sci Technol. 2021;10(3):5. |