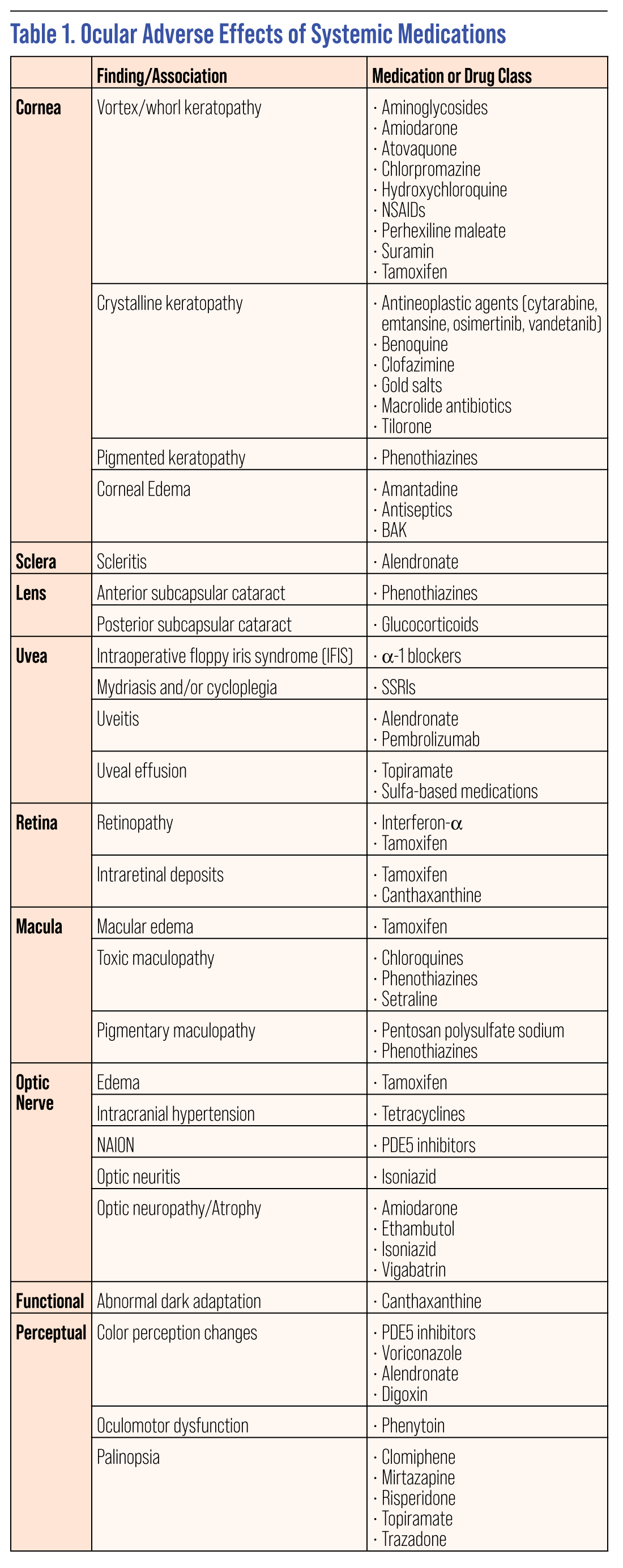
The list of systemic drugs that may have ocular effects is enormous. While perusing a patient’s medication list and considering all the possible effects can be daunting, it is important to be watchful and educate the patient about any concerning ocular complications that could potentially arise. However, rather than the typical “this medication can cause these effects” mentality, it’s often more clinically relevant to think of it the other way around: “With this clinical finding, could one of the patient’s medications be causing the problem?” This sort of effect to cause rather than cause to effect thinking can help you when you’re confronting a clinical finding in your chair and need to think backwards to determine the source.
Here, we’ll touch on some of the more commonly encountered ocular side effects related to systemic meds, grouping the findings by ocular location or function to help allow that “retrace your steps” approach to connecting presentations to medications.
 |
|
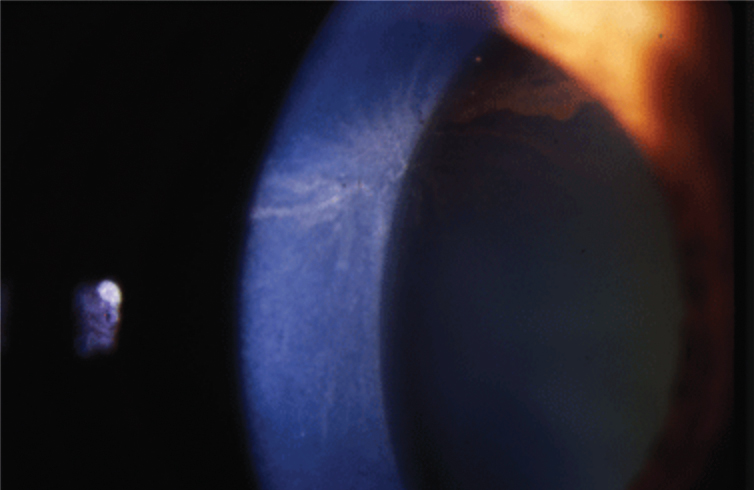
Fig. 1. Amiodarone causes corneal whorl keratopathy, or corneal verticillata, visible at the level of the basal epithelium. Photo: Jay Pepose, MD, PhD. Click image to enlarge. |
Cornea
Some of the most readily apparent effects of systemic meds appear in the cornea, given the structure’s ease of observation to patient and doctor alike. Many drugs allow drug penetrance into cells with the potential for phospholipid accumulation intracellularly. This manifests in the eye as vortex (or whorl) keratopathy (Figure 1). The finding starts at approximately three weeks and is generally reversible within months of discontinuation of the medicine. Vortex keratopathies rarely have any effect on acuity but can cause blurry vision or, on rare occasions, even corneal ulceration. Drug-related keratopathies generally resolve with discontinuation of the offending agent.
Drugs causing vortex epithelial deposits include amiodarone, hydroxychloroquine, chlorpromazine, tamoxifen, nonsteroidal anti-inflammatory drugs, atovaquone, suramin, perhexiline maleate and aminoglycosides.1
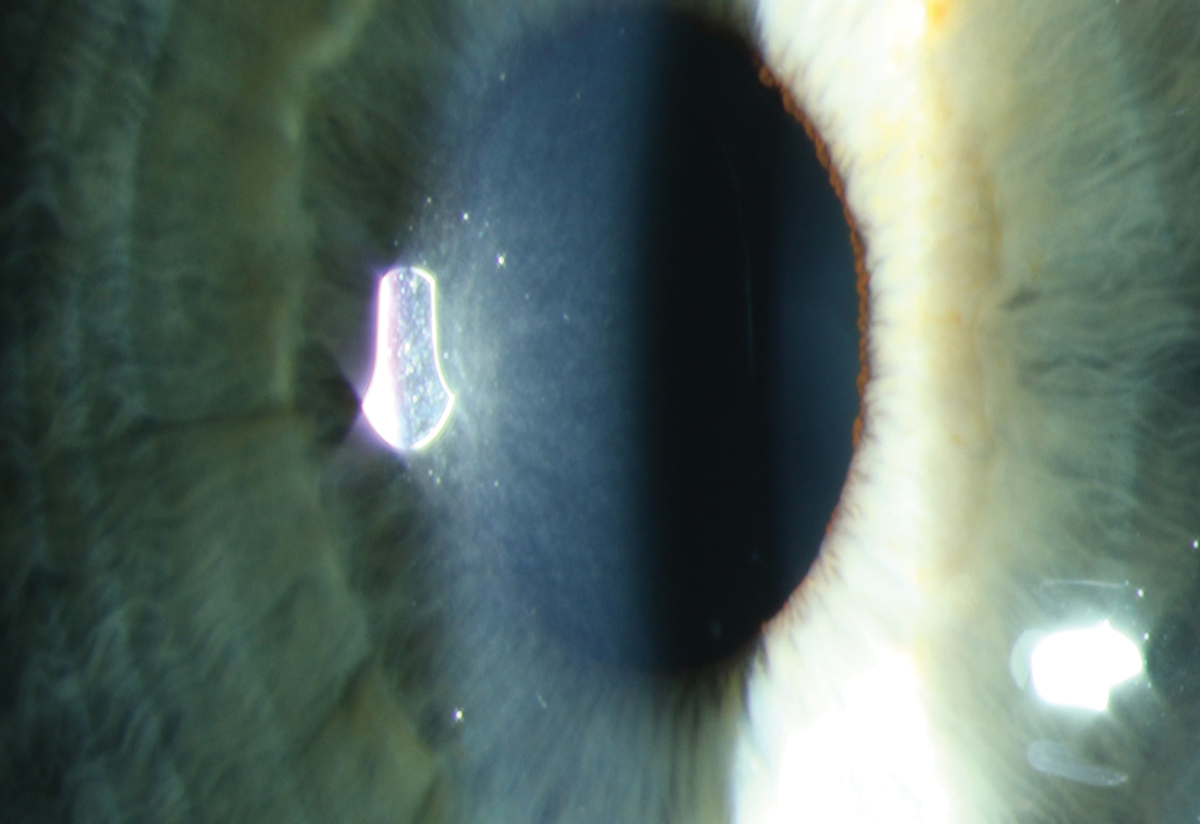
Similarly, many drugs are subject to lysosomal sequestration or drug precipitation, which can lead to a crystalline keratopathy. These often begin as a diffuse punctate subepithelial deposit, which may become more linear. In contrast to whorl forms, crystalline-like keratopathies are more likely to elicit a decrease in acuity, especially when caused by antineoplastic agents.
Inciting agents include benoquine, tilorone, macrolide antibiotics, clofazimine, gold salts and multiple antineoplastic agents, including vandetanib, osimertinib, cytarabine and emtansine (Figure 2).1 While discontinuing the offending agent may be impossible, it’s important to identify the correlation and communicate with the prescribing physician to consider titrating to a tolerable dose.
While any topical ophthalmic drug containing the common preservative benzalkonium chloride (BAK) can independently elicit corneal edema, as can several topical antiseptics, it is a very rare complication of systemic medications. Most notably, amantadine—an anti-Parkinson’s drug—has been demonstrated to reversibly provoke corneal edema in rare cases.
 |
|
Fig. 2. Early subepithelial refractile deposits in a patient on macrolide therapy for chronic obstructive pulmonary disease. Photo: Sara Weidmayer, OD. Click image to enlarge. |
Lens
Early cataract formation is the most likely drug-related effect we commonly see in practice.
Phenothiazines (chlorpromazine, thioridazine). This is a group of psychotropic medications used to treat serious mental disorders including schizophrenia and psychoses. These drugs, specifically chlorpromazine and thioridazine, are associated with cataract formation in the anterior subcapsular layers and are nearly always associated with pigment accumulation.2 Over time, this pigment can become arranged in a stellate pattern, but is rarely associated with visual impairment.3 These opacities are rarely bilateral but are typically asymmetric when they are, and are not likely to reverse with cessation of the medication.4
Glucocorticoids. These are a group of corticosteroid hormones used to reduce inflammation and suppress the immune system. Whether systemic, topical, inhalant, nasal spray or injected, they can produce posterior subcapsular cataract (PSC) in both adults and children, and do appear to be dose- and time-dependent. While the lenticular changes may progress or remain stationary, they will rarely reverse with discontinuation of the medication. The cataract typically begins as a PSC but can gradually progress to appear similar to other types, including senile cataract.2 Patients on corticosteroids should be educated on the possibility of cataract formation and have routine eye evaluations.
 |
|
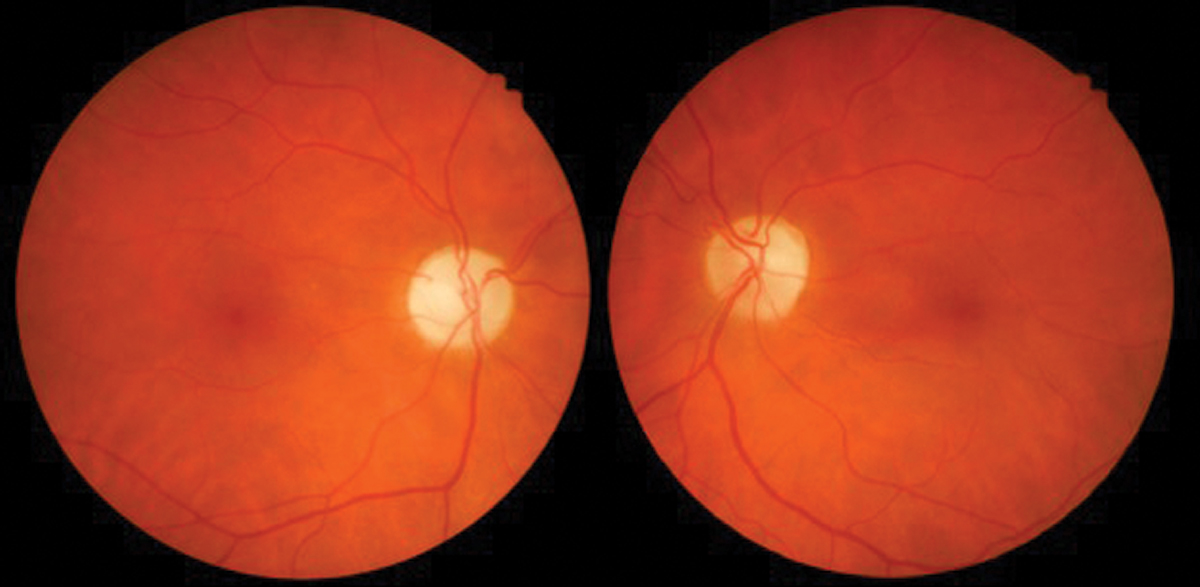
Fig. 3. Interferon retinopathy with cotton wool spots. Photo: Sara Weidmayer, OD. Click image to enlarge. |
Retina
Access to the vasculature makes the retina fertile ground for complications of systemic med use.
Interferon (Intron A, Roferon-A). The interferons (IFNs) are glycoproteins classified as cytokines and used primarily for hepatitis C virus (HCV). Specifically, INF-α used to treat HCV that is associated with ocular manifestations.
The most common finding is INF-α associated retinopathy, predominantly with cotton wool spots and flame-shaped hemorrhages in the posterior pole radiating from the optic nerve (Figure 3). These findings rarely cause significant visual disturbance and may go undetected due to lack of patient symptoms. Risk factors include type 2 diabetes, systemic hypertension, increasing age and duration of treatment.
Retinal changes usually occur by week 12 of therapy but can take as long as 28 weeks to manifest. Typically, the retinopathy will resolve on its own, even with the continued use of the medication. Patients should have a baseline dilated fundus exam prior to initiating INF-α therapy, then follow-up every three to six months throughout the course of treatment. The finding of retinopathy is not typically a reason to discontinue treatment unless there’s associated acute vision loss, and the retinopathy most often resolves less than one month after completion of treatment.5
Tamoxifen (Soltamox). This antiestrogen medication is used to treat estrogen receptor–positive breast cancer, ovarian cancer, pancreatic cancer and malignant melanoma.6 The retinal findings most often associated with it include small refractive bodies in the inner retina, which appear like dot-like yellowish deposits surrounding the macula.2 Patients may also be at risk for development of pseudocystic cavitary spaces—without macular edema—which may only be visible on SD-OCT imaging.
The OCT appearance of the condition appears very similar to that of idiopathic macular telangiectasia type 2 and has been found to occur early in treatment, at low doses of the drug; it is irreversible.7 There is also a more severe form of retinopathy that can include cystoid macular edema, retinal hemorrhages and optic nerve edema, and is associated with vision loss. This more acute form of toxicity is most likely to occur only after a few weeks of starting therapy whereas the less severe form usually occurs after more than one year of treatment, when a total of more than 100 grams of the drug has been dosed.6
Patients should have a baseline eye exam and yearly screening with a dilated fundus examination and SD- OCT imaging. If ocular manifestations are present, it is recommended to consult with the patient’s oncologist as a change in dosage or discontinuation of the drug may be indicated.7
Canthaxanthine (Orobronze). A naturally occurring carotenoid whose oral consumption may cause bronzing of the skin, canthaxanthine is sold over the counter as a tanning agent. This substance has been FDA approved for use as an artificial food coloring agent but not for any other use.
Orobronze deposits in all layers of the retina with a preference for the more superficial layers and will appear as very small, gold-like particles near the macula.6 The associated retinopathy does appear to be dose-dependent, and is found in 50% of patients who have taken at least 37 grams and nearly 100% of those who have consumed at least 60 grams of the drug. Most patients will be asymptomatic; however, occasionally it can cause abnormal dark adaptation. The retinal deposits do resolve with the discontinuation of the drug.8
 |
|
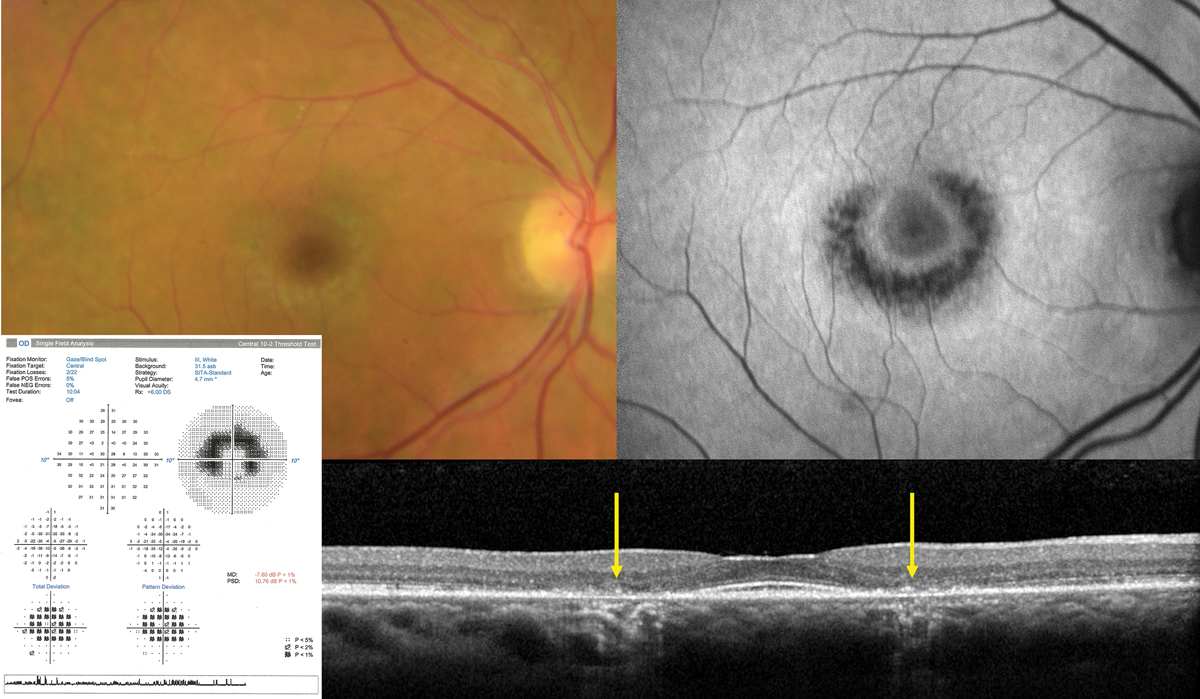
Fig. 4. Classic advanced hydroxychloroquine-induced toxic maculopathy showing parafoveal RPE/outer retinal loss (yellow arrows on OCT), hypoAF on FAF imaging and corresponding paracentral visual field defects. Photo: Sara Weidmayer, OD. Click image to enlarge. |
Macula
Here we encounter some of the heavy-hitters most familiar to optometrists, especially Plaquenil, with which we have a long history.
Hydroxychloroquine (Plaquenil). The choloroquines are a class of chemotherapeutic agents and a mainstay as both treatment and prophylaxis against malarial disease and Q fever. Hydroxychloroquine is effective in managing various autoimmune conditions, including rheumatoid arthritis, Sjögren’s, lupus and porphyria cutanea tarda.
The ophthalmic complications primarily lie in its ability to produce a toxic maculopathy in a dose-dependent manner. Findings include a bull’s-eye maculopathy in the late stages, associated with more subtle findings on OCT, fundus autofluorescence (FAF), multifocal electroretinogram (mfERG) and threshold perimetry findings in the early stages (Figure 4).
Monitoring should include a baseline exam within one year of initiating therapy, then biannual exams after five years of use including OCT and 10-2 threshold visual fields. FAF and mfERG may have utility in early detection. Early findings in OCT may show disruption of the outer ellipsoid zone and parafoveal thinning of the outer nuclear layer, which may progress to “flying saucer” sign with advanced toxicity.
FAF may demonstrate early patchy hyperautofluorescence (hyperAF) circumferentially around the fovea, which may coalesce to a ring in advanced disease. mfERG studies are sensitive but widely variable and are of limited value in isolation. Threshold visual fields will similarly show patchy shallow depressions circumferentially about the fovea which will coalesce into continuous, deeper scotomas in advanced disease.
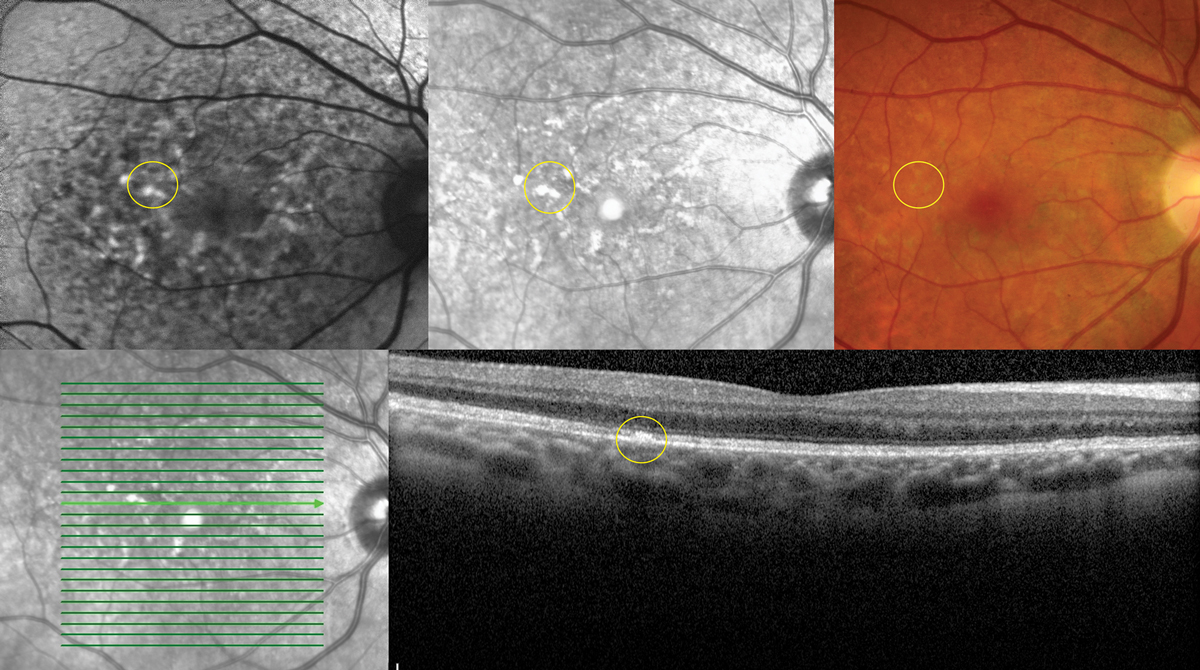 |
| Fig. 5. Multimodal imaging demonstrating collocated yellow deposits, RPE thickening, near infrared hyper-reflectance and hyperAF on FAF imaging in advanced Elmiron maculopathy. Photo: Sara Weidmayer, OD. Click image to enlarge. |
Of note, Asian patients tend to have defects more peripherally in the macula and should be evaluated with a 24-2 visual field. The chance of retinal toxicity increases with time and has as high as a 20% risk at 20 years.9 Concurrent use of tamoxifen increases the risk of maculopathy fivefold.
Pentosan polysulfate sodium (Elmiron). PPS is an oral medication approved for the relief of bladder pain or discomfort associated with interstitial cystitis/painful bladder syndrome.
A unique pigmentary maculopathy was only recently elucidated in 2018 and has subsequently been bolstered by larger population studies. Current evidence suggests a dose-dependent pattern of damage with caution in anyone with a cumulative exposure of greater than 500g. Color fundus photos, OCT, FAF and near-infrared reflectance (nearIR) imaging are key to detection. Fundus evaluation will demonstrate yellow deposits at the level of the retinal pigmented epithelium (RPE), which correlate to hyperreflective focal RPE thickening via OCT. Similarly, these deposits correlate to hyperAF on FAF and hyperreflective patches on nearIR imaging, which show highly disorganized patterns and irregularity in excess of what might be predicted by fundus evaluation.
A hyperAF halo may be evident in the peripapillary region (Figure 5).9 The clinical course and endpoints are unknown, but maculopathy can evolve after discontinuation and patients should be followed at regular intervals.
Phenothiazines (multiple trade names). This drug class encompasses a larger group of antipsychotic agents in which thioridazine and chlorpromazine are the most prescribed. They have been largely supplanted by newer medications— especially in chronic therapy—but are still commonly used.
In contrast to most toxic maculopathies, the macular toxicity seen here is more dependent on daily doses rather than a cumulative one. The mechanism of damage is not completely understood, but they are thought to disrupt rhodopsin synthesis.11
Onset of visual symptoms typically occurs within weeks of initiating therapy in doses greater than 800mg daily of thioridazine and 1200mg daily of chlorpromazine. In the early stages, course granular pigmentation occurs, followed by patchy RPE loss with a characteristic ovoid shape extending to the midperipheral retina. In late stages, widespread areas of depigmentation and hyperpigmented plaques are visible, followed by geographic atrophy.
At most doses, benign pigmented corneal and anterior lens opacities can also occur. OCT can reveal photoreceptor loss, which may span the fovea extending far into the arcades. There is typically some measure of visual recovery upon discontinuation.12 In standard doses, routine exams are sufficient for monitoring, while symptomatic individuals should be examined within the week. Alternate meds should be discussed with the prescribing practitioner at the first sign of toxicity.
Sertraline (Zoloft, Celexa, Lexapro, Prozac, Paxil). This a commonly prescribed selective serotonin reuptake inhibitor (SSRI) for depressive and anxiety disorders and more infrequently for bulimia nervosa and anorexia nervosa.
While the link between sertraline and maculopathy has yet to be firmly established, there are multiple case reports describing acute-onset bilateral maculopathies in otherwise healthy eyes following initiation of therapy at standard doses, whose findings closely mimic those in advanced hydroxychloroquine toxicity.13 While maculopathies appear infrequently, the reported cases are profound, so be aware of this potential adverse effect given the widespread use of this medication.
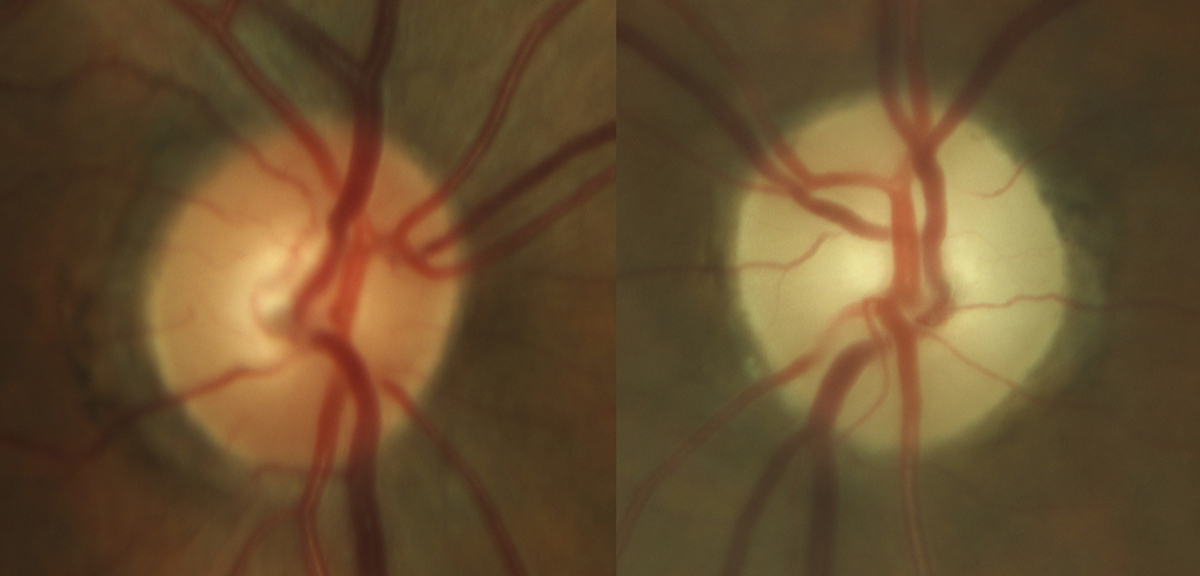 |
Fig. 6. Late stage, severe optic atrophy OS in a patient on chronic amiodarone therapy. Photo: Sara Weidmayer, OD. Click image to enlarge. |
Optic Nerve
As we encounter neuro-ophthalmic involvement, the potential for serious vision loss escalates.
Phosphodiesterase Type 5 (PDE5) Inhibitors (Viagra, Levitra, Cialis). This class of drugs is used to treat erectile dysfunction (ED). There has been an association between PED5 inhibitors and non-arteritic anterior ischemic optic neuropathy (NAION); however, there has been no evidence confirming a cause-effect relationship. The clinical picture of NAION in patients taking these medications is the same as one that occurs spontaneously: edematous and hyperemic optic disc, relative afferent pupil defect and painless loss of vision.
It’s important to note that most patients who require the use of ED medications have the same risk factors (older age, vascular risk factors such as hypertension and diabetes) for NAION, which makes it very difficult to establish a definite association between the two.2 It is recommended that these drugs be avoided in patients who have experienced NAION in one eye, as they may be more likely to develop the condition in the same or fellow eye.6
Amiodarone (Pacerone). An anti-arrhythmic drug, amiodarone is one of the most effective medications available to treat various cardiac arrhythmias.
While the exact mechanism is unknown, there is a probable association between amiodarone use and optic neuropathy. However, note that the neuropathy secondary to amiodarone use differs from typical NAION in that the condition is bilateral, associated vision loss progresses slowly over several months and the degree of vision loss tends to be less severe. The disc edema is also much slower to improve when compared to NAION, which can help distinguish between the two.
Patients on amiodarone should have a baseline exam with follow-up every 12 months, or immediately should visual symptoms occur.
If optic neuropathy is suspected, it’s recommended that the decision to discontinue the medication be discussed with that patient’s prescribing cardiologist. Once the drug has been discontinued, it may take several months for the edema to resolve, ultimately leaving the patient with optic atrophy (Figure 6).6
Vigabatrin (Sabril). An anticonvulsant used in the treatment of seizures, vigabatrin is most often used to manage drug-resistant partial seizures and infantile spasms.
It has been associated with visual field loss and optic nerve atrophy, although the exact mechanism is unknown. These complications are typically irreversible and occur in approximately 45% of patients treated with this medication. Exam shows a bilateral, symmetric, concentric peripheral field defect with no central involvement. Retinal nerve fiber layer (RNFL) analysis with OCT shows thinning, with corresponding pallor of the optic nerves.
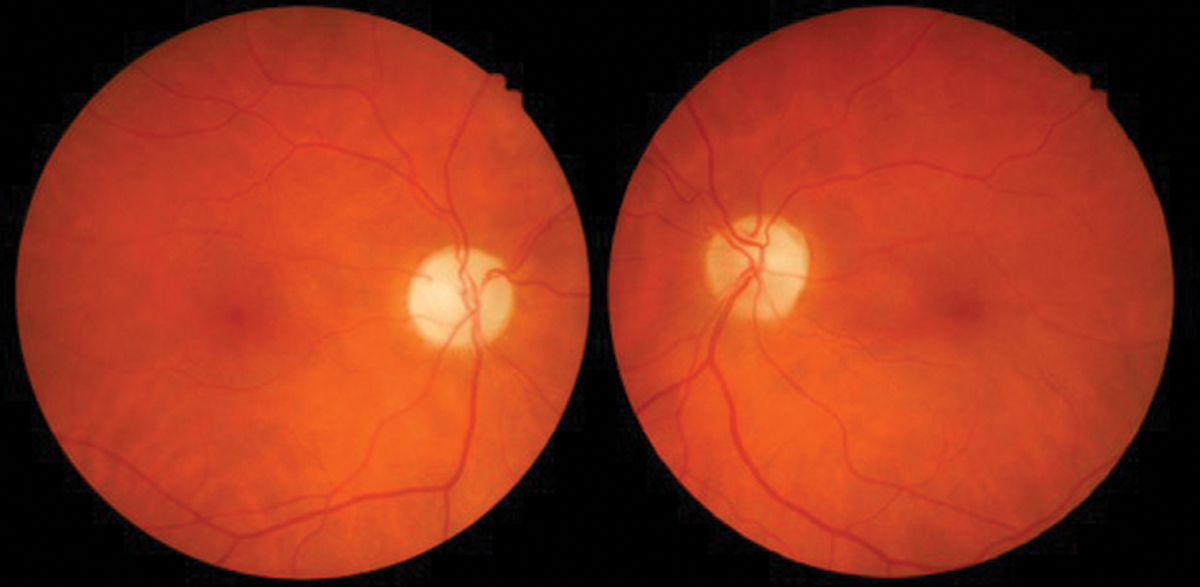 |
|
Fig. 7. Optic neuropathy with pallor OD>OS due to ethambutol. Photo: Sara Weidmayer, OD. Click image to enlarge. |
Even in severe case of visual field loss and optic atrophy, visual acuity can be normal and the patient asymptomatic. The damage does appear to be dose-dependent and irreversible with the first signs of RNFL thinning showing around 1000 grams cumulative dose and worsening as that dose increases.14
Patients should have a comprehensive eye exam within four weeks of starting the medication, every three months during treatment and three to six months once treatment has been discontinued. If vision loss is confirmed or suspected, the prescribing physician should be consulted and the medication should be discontinued.15
Ethambutol (Myambutol). This drug treats pulmonary tuberculosis and there is significant evidence of optic neuropathy associated with its use (Figure 7). The condition is typically bilateral but can be asymmetric or unilateral.
The most common clinical findings include a decrease in visual acuity, color vision loss or central scotoma on visual field testing. Ethambutol may also affect the optic chiasm and cause bitemporal visual field defects. The adverse ocular effects appear to be related to dose, with those taking 60-100 mg/kg/day at the highest risk of 50%. At the recommended dose of 15mg/kg/day or less, the risk goes down to 1%.
Patients prescribed this medication should be made aware of the potential for severe, irreversible vision loss and a baseline exam, including visual field and color vision testing should be performed. RNFL OCT analysis is also recommended, as it could possibly detect early stages of toxicity. If patients are taking greater than 15mg/kg/day, it is recommended they have monthly follow-ups. Those taking lower doses may need to be followed closely if they have any conditions that may put them at higher risk for toxicity, such as diabetes, chronic renal failure or alcoholism.
The medication should be discontinued, at the discretion of the treating physician, if there is any evidence of vision loss, visual field or color vision defect.6
Isoniazid (Nydrazid). Another pulmonary tuberculosis drug, this one is commonly prescribed in combination with other meds due to concern of drug resistance to monotherapy. Isoniazid has also been associated with optic neuropathy; therefore, when used with ethambutol, it can be difficult to determine the causative agent.
Optic neuropathy from isoniazid tends to occur less frequently, is less severe and is reversible with medication discontinuation.6 Rarely, the drug has been associated with optic neuritis. Visual symptoms are like those seen with other optic neuropathies and include decreased vision, loss of color vision and either a bitemporal or centrocecal visual field defect. The optic nerves appear hyperemic with blurred margins, though rarely may appear normal. The condition is reversible, and improvement can begin as early as four days after discontinuing the medication, with complete recovery taking anywhere from four to six months. Continuation of treatment may lead to optic atrophy.16
Tetracyclines. Part of a group of commonly prescribed antibiotics that also includes doxycycline, minocycline and oxytetracycline, these meds are used to treat conditions like acne, rosacea, blepharitis and dry eye syndrome.
While there is currently no evidence that they cause intracranial hypertension, they have long been associated with it. Patients with this condition most often present with headache, pulsatile tinnitus, transient visual obscurations or diplopia.
Exam findings include bilateral optic disc edema and possibly decreased visual acuity. While obese women in their childbearing years are at higher risk in general for the development of idiopathic intracranial hypertension (IIH), when associated with tetracycline use the condition tends to occur in even younger age groups (teens) and patients are less likely to be obese.
Average duration of antibiotic use before symptom onset can range from 14.4 to 18.9 weeks.17 IIH is a diagnosis of exclusion and additional testing, neuroimaging and cerebrospinal fluid evaluation must be performed for confirmation.18 The condition does tend to improve with discontinuation of the medication, and recurrence—while rare—is typically less severe than the original presentation.17
It is recommended that patients taking tetracyclines be educated about the possible side effects and have an eye exam after one month of treatment. Patients who experience any of the common IIH symptoms should be instructed to seek prompt care with an eye care provider.19
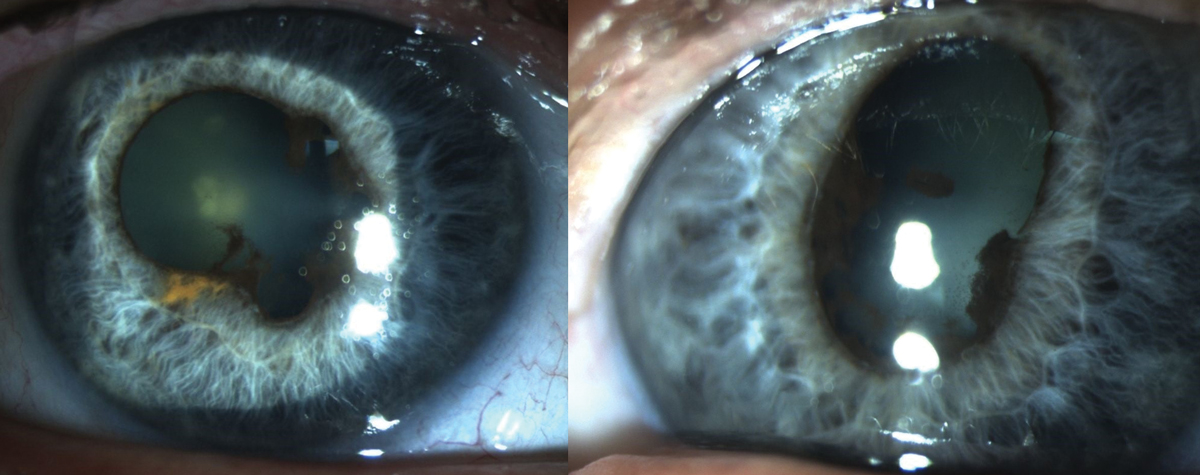 |
|
Fig. 8. Findings due to chronic anterior uveitis associated with Keytruda use. Photo: Sara Weidmayer, OD. Click image to enlarge. |
Inflammatory Changes
For the remainder of our discussion, we’ll shift emphasis from anatomical changes to those affecting multiple structures and/or functions, beginning with the effects of inflammation.
Alendronate (Fosamax, Actonel, Boniva, Reclast, Prolia, Evista, Duavee). Bisphosphonates inhibit bone resorption and are most used to treat or prevent osteoporosis in post-menopausal women.
The most common ocular side effect is uveitis or scleritis. Patients with uveitis will typically present with severe pain, redness, sensitivity to light and decreased vision. There will be cell in the anterior chamber and occasionally keratic precipitates. Scleritis will cause a severe deep pain, often that awakens the patient at night. The sclera can have a deep purple hue with vessel engorgement, and scleral edema and thinning can occur.
These conditions can be unilateral or bilateral and the onset of symptoms is two days to two weeks after starting treatment. In almost all cases of inflammation, the alendronate must be discontinued for the condition to fully resolve and reinitiating any bisphosphate prompts recurrence of the inflammation.6,20
Pembrolizumab (Keytruda). This is a cancer immunotherapy drug that works by increasing the natural immune system’s ability to detect and fight cancer cells. Due to the increased immune response, this drug has been associated with ocular inflammation, specifically anterior uveitis or panuveitis (Figure 8).
Classic uveitis symptoms include pain, redness, photophobia and decreased vision present, typically with mild to moderate cells in the anterior chamber. It is possible, however, to have a more severe reaction with vitreous involvement. The condition is almost always bilateral and will occur within the first six months of therapy. Most patients respond well to traditional topical uveitis treatment; in rare cases, long-term inflammation and/or severe vision loss have been reported.
Patients on pembrolizumab should have a baseline exam and be educated on ocular side effects. Routine monitoring once treatment has been started is acceptable but may not be necessary unless patients become symptomatic.21
 |
| Click table to enlarge. |
Mechanical Changes
Ocular effects in this category can complicate surgical procedures and, in severe presentations, expose the patient to risk of angle closure.
Topiramate (Topamax). An oral sulfamate originally approved as an anticonvulsant, topiramate has more recently been expanded for use for migraines, weight loss, depression and ethanol dependence.
The drug may induce uveal effusions, which result in ciliary body edema and forward rotation of the lens-iris diaphragm and angle closure glaucoma. Myopic shifts average under one diopter but have been reported at greater than eight.
Adverse events are rare, but typically occur within two weeks of starting the medication. Much rarer cases of ciliary body effusion have been reported with other sulfa drugs including acetazolamide, sulfamethoxazole, promethazine, spironolactone, isosorbide dinitrate and bromocriptine.22 Isolated cases have also been reported with tetracyclines, bupropion, oseltamivir, duloxetine and aspirin.
Recognition of the inciting medication is critical, as the treatment differs from a primary angle closure and includes the use of cycloplegics and a topical steroid with discontinuation of the inciting medication.
Alpha-1 blockers (Flomax, Uroxatral, Rapaflo). These treat symptoms of benign prostatic hyperplasia (BPH).
The presence of alpha-adrenoceptors in mediating the contraction of the dilator pupillae muscle leads to a generalized miosis and the potential of intraoperative floppy iris syndrome (IFIS). This condition shows a billowing iris, rapid progressive intraoperative miosis and protrusion of iris tissue through surgical incisions. The effect tends to occur in as little as two weeks after initiation of therapy and remains even when discontinued. Severe IFIS occurs in roughly one-third of those taking Flomax and Rapaflo, while the effect is less pronounced with Uroxatral.23
Although the challenges surrounding IFIS cannot be eliminated with discontinuation of the drug, the intraoperative risks can be mitigated with intracameral epinephrine, preoperative atropine, intraoperative trypan blue and iris retractors. As such, simply recognizing the risk and appropriate preoperative planning should minimize IFIS.
Perceptual Changes
These adverse effects may be particularly troubling to patients without necessarily representing high-risk events that jeopardize eye health.
Color Changes (multiple classes). PDE5 inhibitors demonstrate mild cross-reactivity with PDE6 receptors in the retina. Changes in color perception are a common side effect, with a blue tint being the most common. The effect occurs in a dose-dependent manner with a 5% chance of side effects at a standard dose. Voriconazole—a common antifungal—has a similar cross reactivity and may elicit similar changes in perception.11 Fosamax and digoxin are commonly associated with a yellow or gold color disturbance.
Palinopsia (multiple classes). Defined as isochromatic afterimages which last following the removal of a stimulus, palinopsia can be caused by a wide variety of medications. Razadone, clomiphene, risperidone, topiramate and mirtazapine have been associated with these manifestations.12 The mechanism of action is unclear, but effects on posterior cortical matter are implicated.
Blurred vision/diplopia (multiple classes). Blurred vision and diplopia can occur with a wide variety of oral meds. The most common classes include alpha-blockers, second generation fluoroquinolones, statins, PDE5 inhibitors and bisphosphonates. The practitioner should investigate each case to rule out the more common etiologies and medications cross-referenced with known side effects.
Functional Changes
Another patient-forward category of effects, these changes are often amenable to mitigation with discontinuation of the drug.
Phenytoin (Dilantin). Phenytoin is a commonly prescribed antiseizure medication and has been associated with oculomotor disturbances including gaze-evoked nystagmus, downbeat nystagmus, periodic alternating nystagmus and partial or complete ophthalmoplegia. These findings can take place in the normal therapeutic range, but are much more common in overdosed/ and toxic levels. Considering the oculomotor findings could suggest a structural brainstem lesion rather than a drug-induced side effect, it is important to review all medications to determine if a full neurologic work-up, including neuroimaging, is indicated. The clinical findings do improve and resolve once the dose is decreased or the drug is discontinued.24
Selective serotonin reuptake inhibitors (Prozac, Zoloft, Paxil, Lexapro, Celexa). SSRIs are psychiatric medications used to treat anxiety disorders.
These drugs are known to cause changes to the accommodative status due to their anticholinergic effects. They cause both mydriasis by stimulating the alpha receptors located on the radial dilator muscle of the iris, and cycloplegia due to the paretic effect on the ciliary muscle. Patients experiencing these effects will likely complain of blur—mostly at near—and occasionally glare. Eye care providers should consider prescribing appropriate spectacle correction to alleviate symptoms. The conditions are reversible once the medication is discontinued.25
Keep a Watchful Eye
As we’re reminded all the time in clinical practice, the eyes are not an island and are very susceptible to medication-induced side effects. Being aware of these possible effects can help providers educate their patients, quickly recognize and mitigate unwanted side effects, and avoid costly and time-consuming workups.
Dr. Case is an attending and residency director at the Fort Wayne VA NIHCS Eye Clinic, an adjunct assistant professor with Indian University and a clinical associate professor with the Michigan College of Optometry.
Dr. Kerns practices at the Wyoming VA Community Based Outpatient Clinic in Wyoming, Michigan, and is on clinical faculty for the Michigan College of Optometry, Illinois College of Optometry and Pennsylvania College of Optometry.
Dr. Weidmayer practices at the VA Ann Arbor Healthcare System and is a clinical assistant professor for the University of Michigan’s Department of Ophthalmology & Visual Sciences. None of the authors have any financial interests to disclose.
1. Raizman MB, Hamrah P, Holland EJ, Kim T, et al. Drug-induced corneal epithelial changes. Surv Ophthalmol. 2017-05-01;62(3):286-301. 2. Li J, Tripathi RC, Tripathi BJ. Drug-induced ocular disorders. Drug Saf. 2008;31(2):127-41. 3. McCarty CA, Wood CA, Fu CL, Livingston PM, et al. Schizophrenia, psychotropic medication, and cataract. Ophthalmology. 1999 Apr;106(4):683-7. 4. Minhas B. The mind’s eye: ocular complications of psychotropic medications. Review of Optometry. 2016 Jan 15; 153(1): 42-49. 5. Rentiya ZS, Wells M, Bae J, Chen KJ, et al. Interferon-α-induced retinopathy in chronic hepatitis C treatment: summary, considerations, and recommendations. Graefes Arch Clin Exp Ophthalmol. 2019 Mar;257(3):447-52. 6. Santaella RM, Fraunfelder FW. Ocular adverse effects associated with systemic medications: recognition and management. Drugs. 2007;67(1):75-93. 7. Doshi RR, Fortun JA, Kim BT, Dubovy SR, Rosenfeld PJ. Pseudocystic foveal cavitation in tamoxifen retinopathy. Am J Ophthalmol. 2014 Jun;157(6):1291-1298. 8. Espaillat A, Aiello LP, Arrigg PG, Villalobos R, et al. Canthaxanthine retinopathy. Arch Ophthalmol. 1999 Mar;117(3):412-3. 9. Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014 Dec;132(12):1453-60. 10. Barnes AC, Hanif AM, Pentosan NJ. Polysulfate Maculopathy versus inherited macular dystrophies: comparative assessment with multimodal imaging. Ophthalmol Retina. 2020 Dec;4(12):1196-1201. 11. Garg P, Yadav S. Ocular side effects of systemic drugs. Era J Med Res. 2019;6(1):1-9. 12. Blomquist PH. Ocular complications of systemic medications. Am J Med Sci. 2011 Jul;342(1):62-9. 13. Ewe S, Abell R, Vote B. Bilateral maculopathy associated with sertraline. Australas Psychiatry. 2014 Dec;22(6):573-5. 14. Clayton LM, Devile M, Punte T, de Haan GJ, et al. Patterns of peripapillary retinal nerve fiber layer thinning in vigabatrin-exposed individuals. Ophthalmology. 2012 Oct;119(10):2152-60. 15. Vigabatrin: Medline Plus Drug Information. US National Library of Medicine. medlineplus.gov/druginfo/meds/a610016.html. Accessed 23 March 2021. 16. Kulkarni HS, Keskar VS, Bavdekar SB, Gabhale Y. Bilateral optic neuritis due to isoniazid (INH). Indian Pediatr. 2010 Jun;47(6):533-5. 17. Orme D, Vegunta S, Miller M, Warner J, et al. A comparison between the clinical features of pseudotumor cerebri secondary to tetracyclines and idiopathic intracranial hypertension. Am J Ophthalmol. 2020; 220:177-182. 18. Thurtell M, Wall M. Idiopathic intracranial hypertension (pseudotumor cerebri): recognition, treatment and ongoing management. Curr Treat Options Neurol. 2013 Feb;15(1):1-12. 19. Kesler A, Goldhammer Y, Hadayer A, Pianka P. The outcome of pseudotumor cerebri induced by tetracycline therapy. Acta Neurol Scand. 2004;110:408-411. 20. Samalia P, Sims J, Niederer R. Drug-induced ocular inflammation. N Z Med J. 2020 Dec 18;133(1527):83-94. 21. Sun M, Levinson R, Filipowicz A, Anesi S, et al. Uveitis in patients treated with CTLA-4 and PD-1 checkpoint blockade inhibition. Ocul Immunol Inflamm. 2020;28(2):217-27. 22. Chen TC, Chao CW, Sorkin JA. Topiramate induced myopic shift and angle closure glaucoma. Br J Ophthalmol. 2003;87(5):648-649. 23. Handzel DM, Briesen S, Rausch S, Kälble T. Cataract surgery in patients taking alpha-1 antagonists: know the risks, avoid the complications. Dtsch Arztebl Int. 2012;109(21):379-384. 24. Praveen-kumar S, Desai M. Ocular motor abnormalities in a patient with phenytoin toxicity--case report and minireview. Clin Neurol Neurosurg. 2014 Dec;127:116-7. 25. Minhas B. The Mind’s Eye: Ocular complications of psychotropic medications. Review of Optometry. 2016 Jan 15; 153(1): 42-49. |

