Annual Retina ReportCheck out the other feature articles in this month's report:Reconsider Your Approach to Diabetic Retinopathy Macula Exam Tips and Tricks Setting Patient Expectations for Anti-VEGF Therapy Tracing Clues Back to Retinitis Pigmentosa |
Geographic atrophy (GA) represents a late stage of age-related macular degeneration (AMD) where there is loss of the photoreceptors, the retinal pigment epithelium (RPE) and the underlying choriocapillaris.
Clinically, this often appears as well-defined regions with greater visibility of the choroidal vasculature in the macula. Atrophic regions may be small and multifocal early in the disease course, and can spare the fovea. As these regions progress, they can expand, taking any shape as they coalesce over time. The presence of extensive drusen or exudative changes may mask smaller atrophic areas, particularly with a light or blond colored fundus. Visual decline from GA typically occurs once these regions are adjacent to or encompass the fovea. However, the atrophy itself need not impact visual acuity directly to impact quality of life issues such as reading and driving.
The prevalence of GA goes up with age and it is slightly less than that of neovascular AMD.1 In the United States, researchers estimate GA’s prevalence is approximately 0.81% having the atrophic form in at least one eye, but increases to 3.5% in patients older than 75.1 Severe vision loss from GA is less common than from neovascular AMD, but researchers say it accounts for between 10% and 20% of all cases of legal blindness from AMD.2 As with all forms of AMD, GA is highest in Caucasians, then followed, in order, by Asian, Hispanic and African populations.3Researchers believe approximately four million Americans and Europeans have late-stage dry AMD. This number is projected to increase as the population ages and the number of people worldwide with AMD may grow to 288 million or more by 2040.4
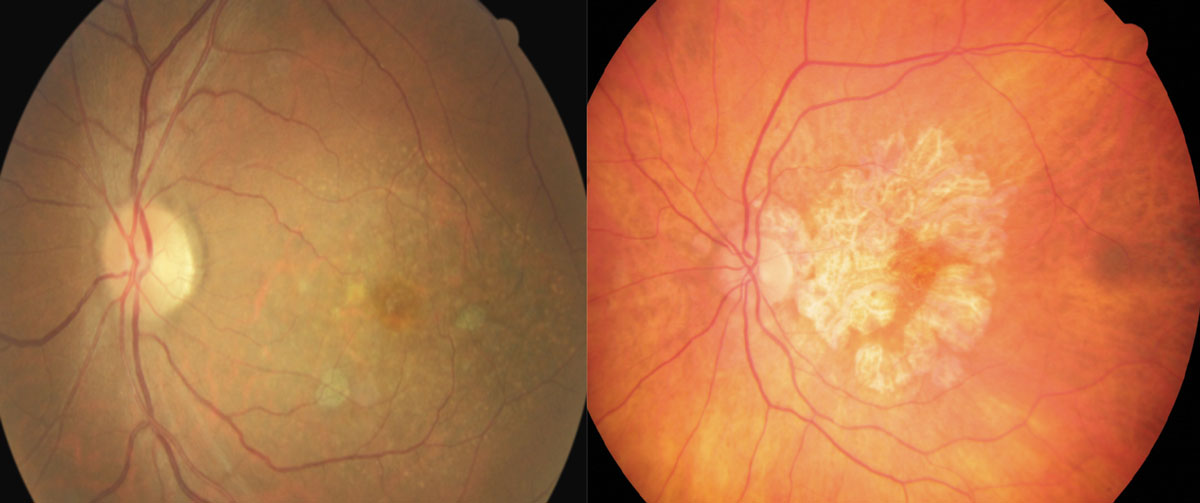 |
| These fundus images show early (left) and advanced geographic atrophy (right). The early patient shows multifocal small extrafoveal regions of GA. In the advanced patient, the lesion has formed an inverted “U” shape almost completely encompassing the fovea, but with apparent foveal sparing. Click image to enlarge. |
Clinically, AMD is generally subdivided into four categories, defined initially by the Age-Related Eye Disease Study (AREDS).5 This scale helps to define where AMD changes from early to late stages (Table 1). Since that was published, other scales have been developed, including the Beckman and Three Continent AMD Consortium scales. Whichever one you use, they all rely on the number and size of drusen as key elements.
Pathophysiology and Progression
GA can be classified into drusen-associated (resulting from non-exudative AMD) and neovascular-associated (resulting from exudative AMD). Although both of these forms of atrophy result in loss of photoreceptors and RPE, the manner by which they do this may differ. Drusen-associated GA refers to the more classically known late stage of dry AMD. Neovascular GA may represent a relatively new form of atrophy. Researchers have only recently recognized it in long-term studies evaluating the treatment of exudative AMD with anti-VEGF injections.6,7
Unlike neovascular AMD, vision loss from drusen-associated GA tends to be more insidious and may go unnoticed until the lesions grow to substantial size, or if the lesion involves the fovea. Rates of GA progression are much slower than rates of exudative AMD. Studies estimate GA progresses anywhere from 0.53mm2 to 2.6mm2 per year, with a median of 1.78mm2.8
Research shows several clinical factors are associated with the progression of GA. These include multifocal lesions at baseline, presence of GA in the fellow eye and the presence of drusen-like deposits interior to the RPE called pseudodrusen.9-11 Patients with GA may still develop choroidal neovascular membranes (CNVM) in other regions, but once the GA has encompassed the majority of macula, risk of CNVM is low.9-11
GA may develop as a result of oxidative stress and accumulation of lipofuscin, structural changes in the retina, RPE and choriocapillaris, and dysregulation in the complement cascade.9-12 It is unknown if these mechanisms are synergistic or if they act independently to lead to the structural and functional changes of this disease.
 |
| In this fundus autofluorescence image of geographic atrophy, you can see darkened regions of atrophy in both the patient’s eyes. Photo: Dr. Laura Addy, OD. Click image to enlarge. |
Oxidative stress on the RPE from accumulation of byproducts, and the aging of the RPE itself is thought to be an important factor in both the development of drusen and in GA.12 The photoreceptors cells are highly metabolic and produce numerous oxidative byproducts which can accumulate in the region where they contact the RPE, futher stressing an already compromised RPE.12
Lipofuscin—another waste product of oxidation—coming from the outer photoreceptors may also play a role in GA by disturbing normal RPE functionality and aiding in the formation of drusen.13 When AMD is present in the eye, the individual RPE cells can migrate out of place, which may also be involved in the progression of the disease to more advanced stages.13
Defects within the complement pathway and alternative complement cascade are also believed to play a role in the in the progression of AMD to GA.14 Genome-wide association studies first brought this link to the attention of the eye research community and it is an emerging area for treatments for AMD.15
The complement system normally manages inflammation and cell death and it is responsible for clearing apoptotic cells and protection against infection. Dysregulation of this pathway in the presence of inflammatory proteins found in AMD may contribute to the cell death in GA.16 The abnormalities can be found in many of the complement factors in the complement pathway, and thus provide multiple therapeutic targets for interruption to prevent worsening of GA.16 Lastly, genetic factors play a role in the development and progression of AMD.17 Known polymorphisms in some genes, including the CFH gene (a complement factor), can be passed down in families, which can increase the susceptibility of patients to develop GA.17
Examination
In monitoring GA patients, optometrists need to establish a baseline and regularly monitor for both changes to the patient’s biological structures and their visual functioning.
For GA specifically, our clinic performs structural imaging with OCT every six months, or in any case of subjective visual changes. Functional tests can be added with patient reported changes in vision as needed. Since GA progresses at different rates for each patient, and even in each eye in each patient, no set protocol exists.
Structural imaging. GA can be imaged using a variety of methods. Color fundus photography is one of the more accessible modes of imaging but newer technologies allow for the capture of even more subtle changes.
Fundus autofluorescence (FAF) relies on short wavelength excitation of lipofuscin found in the RPE. In the case of GA, FAF shows large areas of hyporeflectance in the regions of RPE loss, often with a perilesional region of hyper-reflectance around the atrophy representing regions of the retina with accumulation of lipofuscin and other autofluorescent byproducts whch are in regions that are progressing. Take these photos early to establish a baseline for reference going forward. Photos with FAF can be repeated yearly.
OCT is also commonly employed to observe areas of GA. OCT angiography (OCT-A) is an even more advanced option. OCT reveals the loss of the outer retinal layers and the collapse of overlying layers in the region of the GA. These often exhibit an increased reflectance of the underlying choriocapillaris and choroid in the absence of the RPE and photoreceptors (i.e., the “waterfall” artifact). This is the easiest way to follow GA patients and it should be repeated at least every six months along with OCT-A to evaluate for neovascularization if available.
Fluorescein angiography has also been used to evaluate GA size and other characteristics, but it is typically more beneficial for detecting neovascularization in AMD. More recent imaging capture techniques, which include near-infrared reflectance and multicolor imaging, offer alternative means of imaging GA that highlight lesion borders markedly in comparison with color fundus photography. As imaging continues to move forward, adaptive optics scanning laser opthalmoscopes are allowing researchers to image individual cells, drusen byproducts and cellular microstructure.18
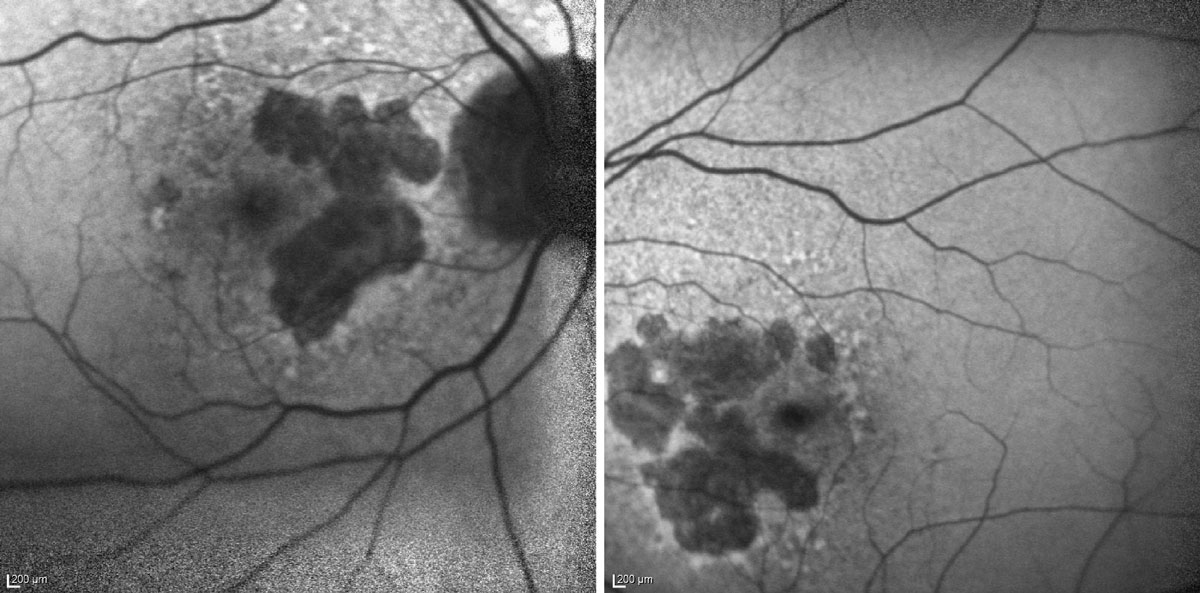
 |
| Here are three types of images of the same patient with subtle juxtafoveal GA. The color fundus photo (left) shows multiple soft drusen but no sharply defined GA lesion. The en face OCT image (center) shows well-defined GA borders. The OCT of the macula (right) reveals a juxtafoveal region of GA showing collapse of the retinal tissue just nasal to the fovea, with loss of the photoreceptors and RPE and revealing an increased signal of the underlying layers. Click image to enlarge. |
Functional testing. Standard automated perimetry (SAP) is not typically of benefit to measure visual dysfunction due to GA alone. However, it may provide a qualitative measure of functional vision if the right strategy is employed for the location of the atrophy. To achieve this, be sure to set the target at the correct size so the patient can view it and, if possible, choose a testing strategy where as many targets are in the testing area as possible. Kinetic targets may be the best option for these cases.
Microperimetry may offer a more appropriate means of monitoring for progression because the regions tested are smaller and more directed, but such instrumentation is not as readily available and would likely require more advanced techniques during testing than is commonly performed with SAP.
It is typical to run a field—such as a Humphrey visual field (HVF) 10-2 or a kinetic strategy—when GA is noted to determine proximity of vision loss to central vision and overall size of any scotoma.
Amsler grid testing is also common, as it can be done by the patient at home with no other special equipment, but it lacks the sensitivity needed to detect small changes in the size of the atrophy.
Multifocal electroretinography (mfERG) also tests retinal functioning. Its use in GA is largely for evaluating retinal function when there is a discrepancy between what clinicians observe structurally and what patients report functionally. For example, a case where the practitioner views the retina as having only a couple small drusen and pigment changes in a patient who is 20/100 or 20/200 could benefit from an mfERG and OCT-A to view changes that are not funduscopically obvious.
At baseline, for a patient with GA, the following tests are typically done in two visits close in time, a field (10-2 or kinetic, sometimes both), an OCT or OCT-A and a photo series with FAF. The patient should be given an Amsler grid to self monitor changes at home. If progression is discovered, or vision is lost, mfERG, microperimetry and FA referrals may be useful for the patient. Otherwise, following with OCT or OCT-A at three-to-six month intervals is sufficient.
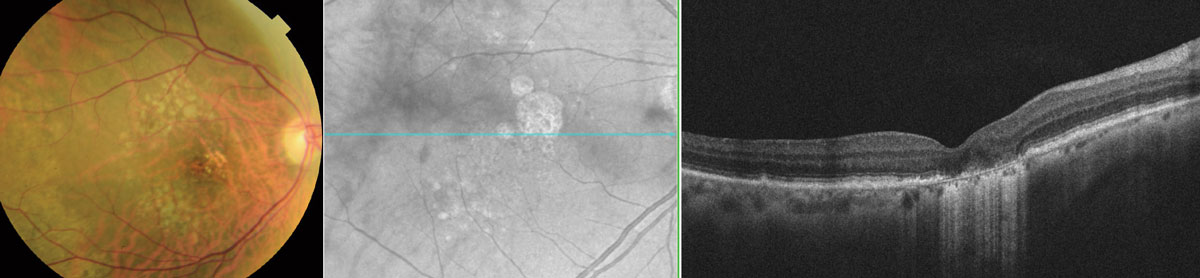
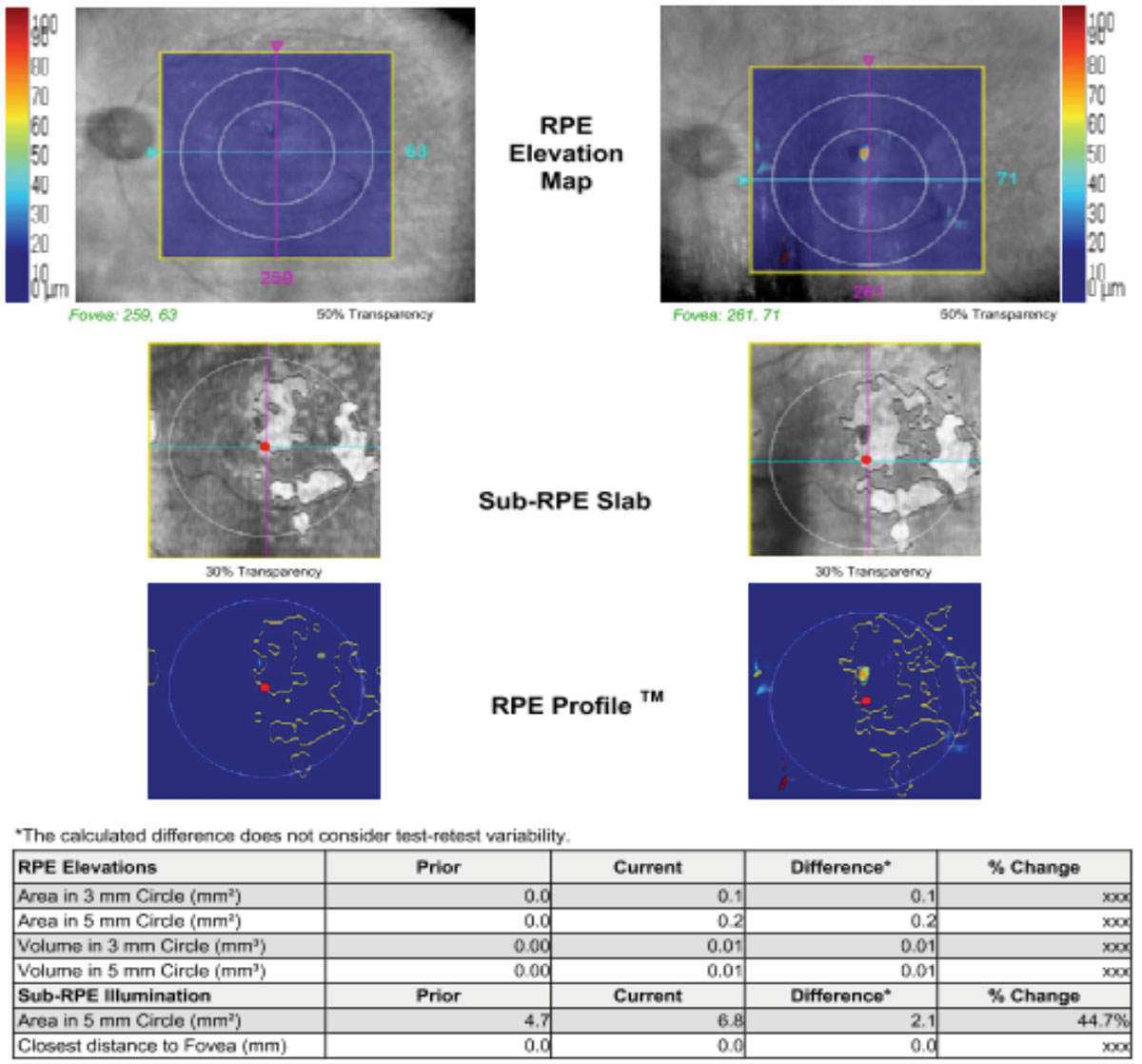 |
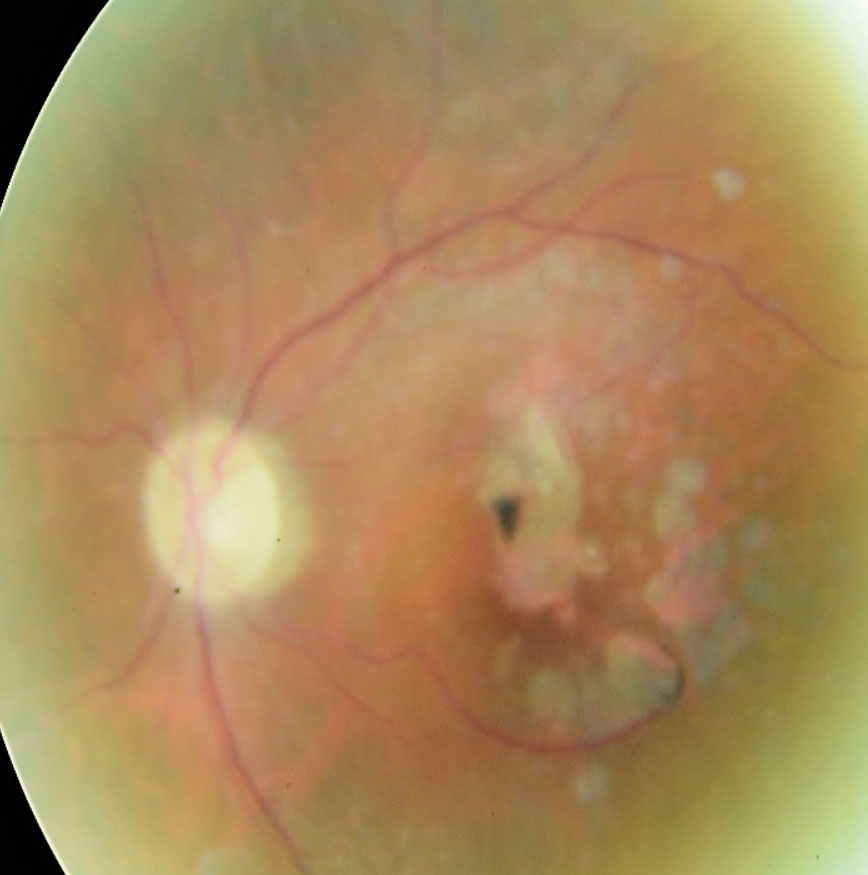 |
| Above, OCT analysis reveals the advancement of GA over time (2014 to 2019) with an increase in area of 44.7% as illustrated in the table. The fundus photo below shows this patient in 2018. Click image to enlarge. |
Treatments
Currently, the FDA has approved no treatments specifically for GA. As with all forms of AMD, the use of high-dose vitamin supplements are recommended for patients with category 3 or 4 level AMD, but research shows supplements make no difference in the progression of existing GA.5
For the most part, management of patients with GA should be aimed at identifying modifiable risk factors (e.g., smoking cessation, nutrition) and monitoring for progression to exudative AMD and/or enlargement of the existing atrophic regions. The patient’s visual demands and limitations should also be considered by the optometrist and visual rehabilitation should be encouraged in advanced cases. Low vision aids can augment visual function for those in need of assistance with everyday life tasks including reading and driving. In cases of larger areas of atrophy outside the fovea, the location of the scotoma caused by the atrophic areas should be considered in association to the patient’s visual demands.
Table 1. Stages of AMD from the AREDS Study | ||
| Category | Drusen | Other Features |
| Grade 1 – No AMD | 5-15 small drusen (<63 microns) | No real pigment changes |
| Grade 2 – Early AMD | Several small drusen, a few intermediate sized (63-124 microns) | Pigmentary changes in one or both eyes |
| Grade 3 – Intermediate AMD | Extensive (20 soft or 65 hard without any soft) intermediate-sized drusen, one large (>125µm) druse | Can have geographic atrophy not affecting the macula |
| Grade 4 – Advanced AMD Grade 4a | Macula-involving geographic atrophy or exudative form with choroidal neovascularization in one eye | |
Advanced AMD in one eye with category 1, 2, or 3 AMD in the fellow eye | ||
| Advanced AMD in one eye and decreased visual acuity (<20/32) secondary to AMD in the fellow eye; however, advanced AMD is not present in both eyes | ||
Clinical trials are currently underway for several investigational biological interventions. One of these treatments is APL-2, a factor that inhibits the complement cascade at C3.19,20 In the early research, APL-2 has reduced the growth of GA lesions (compared with sham).
Zimura (avacincaptad pegol, Iveric Bio) is another drug that may be available for use in the near future. It is a complement factor 5 inhibitor. Its Phase IIb results found a mean reduction in atrophy of 27%.21
Additional clinical trials for GA drugs include ongoing evaluation of 40mg Oracea (doxycycline, Galderma) for GA.22 This 31-month study, with observation and treatment phases, is ongoing.22
With the introduction of Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics) RPE-65 gene therapy for retinitis pigmentosa, as well as Leber congenital amaurosis, research has turned its attention to gene therapy for other retinal pathologies, including GA.
For instance, Gyroscope Therapeutics, a UK-based company, is developing a formulation called GT005 to stimulate the production of complement factor I (CFI) with the hope that it can restore balance to the complement system and slow the progression of GA.23 A reduction of CFI in the serum is a factor that can contribute to GA.23 Patients in the ongoing Phase I/II clinical trial, known as the FOCUS study, receive a single dose of GT005 that is surgically delivered to the suprachoroidal space.23 The company is preparing to move into its proof of concept Phase II clinical program later this year.23
Patient Education
Given the information above, when an optometrist examines a patient who shows early signs of AMD or AMD that has progressed toward GA, it’s time for an important conversation. This conversation should have three parts. First, the optometrist must explain the data. Second, the modifiable risk factors and low vision need to be discussed. Lastly, the importance of follow-up should be emphasized.
Specifically, the patient should be shown their fundus photograph and areas of atrophy, and drusen and pigment changes should be explained if possible. The OCT can also aid in this discussion as it shows the drusen as areas of buildup that affect vision. Areas of atrophy can be explained as scars.
Most patients have heard of AMD and many will be frightened by the diagnosis. It is important to share data with the patient so they understand where they are in the disease process. If FAF is available, those images can be easy enough for the patient to understand.
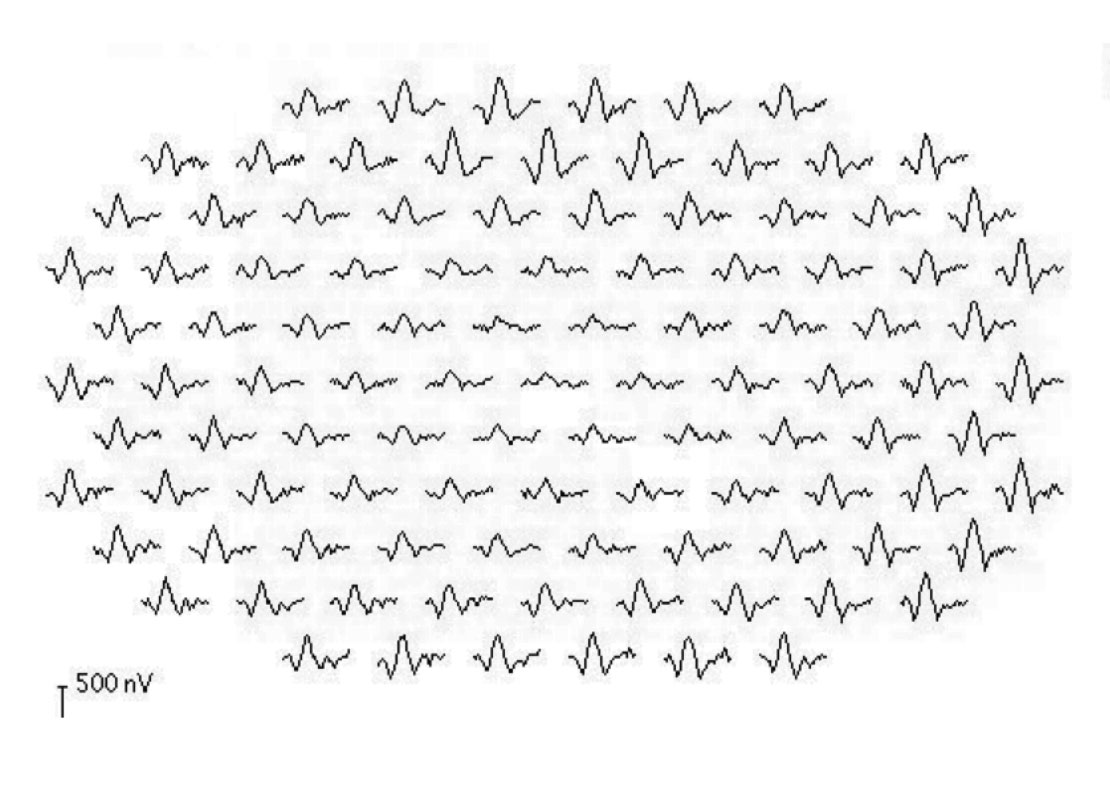 |
| This mfERG shows a patient with central atrophy. Note the reduction in the size of the peaks for the central waveforms. Click image to enlarge. |
Most patients ask if they are going to lose all their vision during this conversation, and even if they don’t ask, they are likely worried about this possibility, so include this element in the conversation. The response should be honest and compassionate. AMD only affects central vision, so all vision will not be lost, but this could affect elements of their life if it progresses.
We tell patients that we don’t know what the future will hold, but there are things they can do to help their eyes. It is better for them to hear the truth from their doctor than to search the internet and potentially find unreliable sources. The discussion should then turn to what the patient needs to do. As there are no FDA-approved treatments for GA, this discussion will involve reducing risk for progression as much as possible. No studies indicate how to do this, but for GA most clinicians believe that reducing the risk factors for AMD could reduce risk of progression. Helpful steps can include smoking cessasion, if needed, a diet filled with green leafy vegetables, AREDS2 vitamins, sunglasses to reduce UV exposure to an already fragile retina and monitoring for changes to neovascular AMD, which can be treated. Also, explain that AMD runs in families, so they should tell their loved ones about their diagnosis.
Low vision aids may also be of use to these patients. In fact, any GA case should be referred to a low vision provider, even if acuity is still adequate, to offer them specialized support. It is an error to wait until the patient is 20/200 to consider a referral to a low vision specialist for these patients. Patients in the 20/50 range can learn how to use devices which will continue to aid them if they progress and early referral for GA patients to low vision can be beneficial.
Lastly, the doctor should discuss how to use an Amsler grid with the patient. While the grid is not sensitive to small changes, it is a marker for larger change in vision and gives the patient some part of their own care. Patients are told if they see a difference in the grid or while reading to please call the office. Patients are typically seen every three to six months otherwise for dilated examination with OCT and OCT-A and occasional HVF. The other tests can be added as needed to monitor changes carefully. If the patient develops neovascularization, they need prompt referral for treatment. It is important for the patient to know that neovascular AMD does have treatments which would need to be initiated as quickly as possible.
Overall, your management of GA should include identifying modifiable risk factors for progression and monitoring visual function and structural changes that may alter the patient’s quality of life, along with a combination of careful examination, regular follow-up and visual aids. The clinician should determine exam intervals based on progressive risk factors, such as the level of vision in the fellow eye, confluent or soft drusen, presence of pseudodrusen, or development of CNV in the fellow eye.
A maximum of six-month intervals is ideal for most patients who are already experiencing evidence of GA, and you can shorten these intervals if patients note any progression or have extensive vision loss.
With any luck, future treatment options will alter the progressive course of this disease and spare visual morbidity. If any of them do pan out, the improved detection techniques optometry has developed—both structural and functional—are likely to remain valuable and perhaps play an even more vital role in AMD management.
Drs. Harrison and Wheat are associate professors at the University of Houston College of Optometry.
1. Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564-72. 2. Rudnicka AR, Jarrar Z, Wormald R, et al. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmol. 2012;119(3):571-80. 3. Klein R, Klein B, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmol. 2006;113(3):373-80. 4. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-16. 5. Chew E, Lindblad AS, Clemons T, Group A-REDSR. Summary results and recommendations from the age-related eye disease study. Arch Ophthalmol. 2009;127(12):1678-9. 6. Daniel E, Maguire M, Grunwald J, et al. Incidence and progression of nongeographic atrophy in the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) clinical trial. Clin Trials. clinicaltrials.gov/ct2/show/NCT00593450. August 21, 2017. Accessed May 25, 2020. 7. Bailey C, Scott LJ, Rogers CA, et al. Intralesional macular atrophy in anti-vascular endothelial growth factor therapy for age-related macular degeneration in the IVAN Trial. Ophthalmol. 2019;126(1):75-86. 8. Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmol. 2018 Mar;125(3):369-90. 9. Marsiglia M, Boddu S, Bearelly S, et al. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(12):7362-9. 10. Fleckenstein M, Schmitz-Valckenberg S, Adrion C, et al. Progression of age-related geographic atrophy: role of the fellow eye. Invest Ophthalmol Vis Sci. 2011;52(9):6552-7. 11. Schmitz-Valckenberg S, Sahel JA, Danis R, et al. Natural history of geographic atrophy progression secondary to age-related macular degeneration (geographic atrophy progression study). Ophthalmol. 2016;123(2):361-8. 12. Ardeljan D, Chan CC. Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retin Eye Res. 2013;37:68-89. 13. Christenbury JG, Folgar FA, O’Connell R, et al. Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmol. 2013;120(5):1038-45. 14. Loyet K, Deforge L, Katschke K, et al. Activation of the alternative complement pathway in vitreous is controlled by genetics in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53(10):6628-37. 15. Fritsche LG, Fariss RN, Stambolian D, et al. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet. 2014;15:151-71. 16. Boyer DS, Schmidt-Erfurth U, van Lookeren Campagne M, et al. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina. 2017;37(5):819-35. 17. Edwards AO, Ritter R, Abel KJ, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421-4. 18. Li Y, Xia X, Paulus YM. Advances in Retinal Optical Imaging. Photonics. 2018;5(2):9. 19. Kassa E, Ciulla TA, Hussain RM, Dugel PU. Complement inhibition as a therapeutic strategy in retinal disorders. Expert Opin Biol Ther. 2019 04;19(4):335-42. 20. Liao D, Grossi F, El Mehdi D, et al. Complement c3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127(2):186-95. 21. IVERIC bio reports positive results for Zimura in dry AMD trial. Clinical Trials Arena. www.clinicaltrialsarena.com/news/iveric-bio-zimura-dry-amd-data/. October 29, 2019. Accessed March 3, 2020. 22. Sadda S, Chakravarthy U, Birch D, et al. Clinical endpoints for the study of geographic atrophy secondary to age-related macular degeneration. Retina. 2016;36(10):1806-22. 23. Kavanagh D, Yu Y, Schramm E, et al. Rare genetic variants in the CFI gene are associated with advanced age-related macular degeneration and commonly result in reduced serum factor I levels. Hum Mol Genet. 2015;24(13):3861-70. |

