26th Annual Glaucoma ReportCheck out the other feature articles in this month's report: |
In the ever-changing health care landscape, optometrists have to constantly adapt to new needs and responsibilities. Glaucoma in particular is emblematic of our evolving role, as advances in technology, pharmaceuticals and research provide opportunities to expand our management of this sight-threatening condition. With the number of glaucoma patients growing steadily, even some ophthalmologists agree that optometrists have a vital role to play in glaucoma management and comanagement.1
To do so, we need to stay on top of improvements in glaucoma care so that we can make the best, evidence-based recommendations for our patients and be a crucial part of a team whose ultimate goal is to preserve sight for years to come. Lowering IOP is still the only known modifiable risk factor for glaucomatous progression, and the average optometrist has a crucial role in determining the best way to do so, whether it be through pharmaceutical intervention or surgical recommendations. With that, any chosen method of IOP control necessitates regular monitoring, which is more available to ODs than ever before, and allows us to effectively manage and comanage these patients with our ophthalmology colleagues.
Here, we take a look at seven vital advancements that have helped put patient care into the hands of the primary care optometrist and how we can use these technologies and techniques to our patients’ advantage while moving the needle on scope of practice expansion.
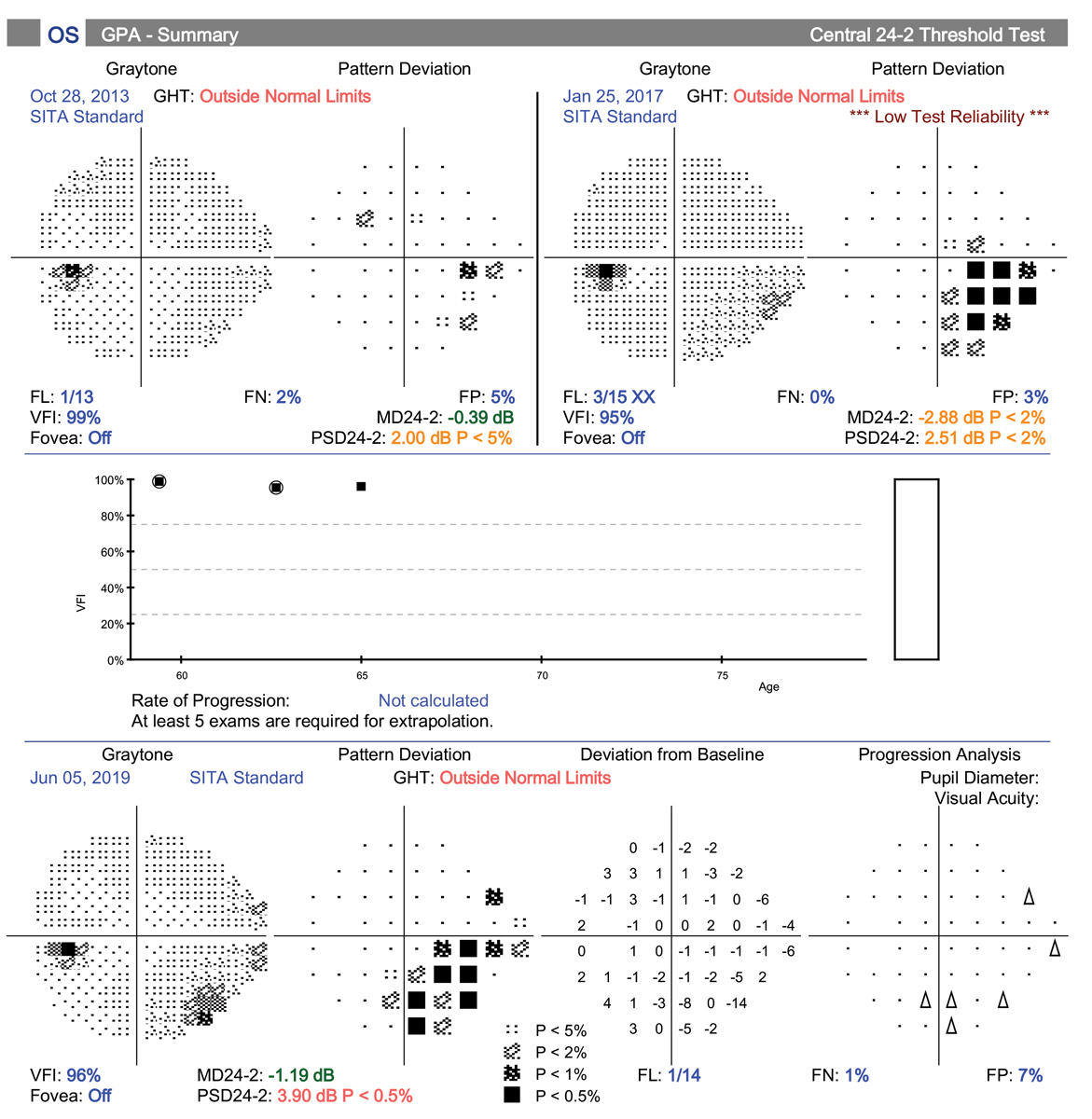 |
This patient’s glaucoma was progressing despite being on two medications. He then developed an early cataract that was causing glare while driving at night. A MIGS device (iStent Inject) was used in conjunction with his cataract surgery. Post-op IOP was 16mm Hg after stopping one of his two meds. Click image to enlarge. |
1. MIGS elevates the role of surgery.
Minimally invasive glaucoma surgery (MIGS) has exploded over the past few years as one of the fastest-growing treatment categories for mild to moderate glaucoma, due to an improved safety profile and decreased risk of complications compared with traditional glaucoma surgery options.2 While efficacy may be modest as a whole compared with trabeculectomy, so too are side effects. Therein lies the category’s chief strength: the risk/benefit balance is decisively in its favor.MIGS is also an excellent option for patients who are noncompliant with drops or have not responded well to procedures such as selective laser trabeculoplasty (SLT).3 Among the top players in this arena currently are the iStent Inject (Glaukos), the Hydrus Microstent (Ivantis), the Xen gel stent (Allergan) and various canal-based procedures such as iTrack (Ellex) and the Omni Surgical System (Sight Sciences). Though ODs should be well-versed in all options, for the sake of brevity we’ll review the iStent and Hydrus here.
The iStent and iStent Inject are tiny trabecular stents made of a biocompatible titanium that provides an excellent safety profile with minimal complications.4 The original iStent device, which was 1mm/0.3mm in size, was implanted manually into the trabecular meshwork (TM) in combination with cataract surgery, with some technical difficulty and learning curve effect.5 The newer iStent Inject boasts an even smaller size (360/230µm) and is now the smallest medical device implantable in the human body. Two stents are present in each preloaded applicator, and they are placed perpendicularly into the TM two to three clock hours apart with relative technical ease for the surgeon.
The two stents placed in this fashion improve access to aqueous collector channels and improve the chances of reaching an episcleral vein, therefore improving the potential for IOP reduction.5 Research shows the original iStent reduces IOP significantly compared with cataract surgery alone, with an even greater decrease with two iStents, as well as allow for a reduction in the number of postoperative IOP-lowering medications needed to achieve goal IOP.6
Postoperative care is similar to that of cataract surgery alone, with no additional visits or medications needed, and is therefore straightforward for the comanaging optometrist.7 However, you may be able to start discontinuing topical glaucoma medication(s) as early as the day-one post-op visit, adding them back as necessary depending on the result.8
Proper patient selection is always the key to success with any procedure. Due to its excellent safety profile, iStent Inject can be confidently recommended for patients with ocular hypertension or mild to moderate open-angle glaucoma (OAG) who have concurrent visually significant cataracts and healthy, open angles, in the absence of inflammation, neovascular glaucoma or other innate or acquired angle abnormalities. They would need to be educated on the risks (nearly none), benefits (potential for reducing drop dependence) and cost, which varies based on insurance coverage and copays.
The Hydrus Microstent (Ivantis) is a small, flexible drainage device inserted in the TM parallel to Schlemm’s canal; this procedure is also combined with cataract surgery. Once inserted, it causes scaffolding of the TM and increases outflow, with increased likelihood of targeting collector channels due to its 90-degree span in the anterior chamber angle. Compared with phaco alone, Hydrus has been shown to reduce IOP another 2.3mm Hg and med use following surgery by 30% through 24 months, with an average reduction in IOP of 7.6mm Hg at two years.9 Post-op care is again similar to phaco alone and can be easily performed by the comanaging OD.
 |
This patient was on two topical meds for her glaucoma, a prostaglandin analog and a fixed-dose combination (brimonidine/timolol), and had SLT within the last year. IOP was 20mm Hg when this disc hemorrhage was noted in the right eye. The patient declined surgical options. Rocklatan (netarsudil/latanoprost) was prescribed (one drop every evening) as a substitute for the PGA. The IOP was reduced to 16mm Hg. Click image to enlarge. |
2. New drugs target different IOP-lowering mechanisms.
After a 15-year drought in the United States without the approval of any glaucoma therapies, several new once-daily topical IOP-lowering medications have become FDA-approved over the past several years. As prescribing IOP-lowering medications is in the domain of the optometrist in nearly every state, this is exciting news that gives us additional treatment options that do not require comanagement.
The first category involves the advent of the long-awaited rho kinase (ROCK) inhibitor netarsudil 0.02%, an entirely new class of glaucoma drug that works by decreasing episcleral venous pressure, decreasing trabecular meshwork resistance and possibly reducing aqueous production.10 It is available packaged alone as Rhopressa (netarsudil 0.02%, Aerie Pharmaceuticals) or in combination with latanoprost as Rocklatan (Aerie Pharmaceuticals), both for once-daily dosing. Netarsudil has been proven effective alone, lowering IOP up to 5mm Hg in its clinical trials, and in fixed combination with latanoprost, it showed a statistically superior IOP reduction over latanoprost and netarsudil alone at every measured time point.11
It has a unique side effect profile, with no serious systemic adverse events reported.10 The main ocular side effect is conjunctival hyperemia, reported in 53% of patients on netarsudil alone and up to 59% of patients using Rocklatan. In clinical practice, however, we have seen that the hyperemia is most noted within the first few days of using the drug, and is worse immediately following administration, and therefore is recommended at bedtime.
Launched in early 2018, Vyzulta (latanoprostene bunod, Bausch + Lomb), a nitric oxide-donating prostaglandin analog, is another relative newcomer to the market. It lowers IOP by a dual mechanism of enhancing uveoscleral outflow while also enhancing TM/Schlemm’s canal outflow by inducing trabecular cytoskeletal relaxation.12 Research shows Vyzulta is more effective than latanoprost alone, with an additional 2mm Hg or more of IOP-lowering ability in 42% of patients, and was proven to have a greater IOP reduction than timolol at nearly all time points measured.13-15 The side effect profile is minimal and comparable to earlier generation prostaglandin analogs.
All three of these drugs are dosed once daily, which is always ideal for compliance. Insurance coverage is improving across the country as well.
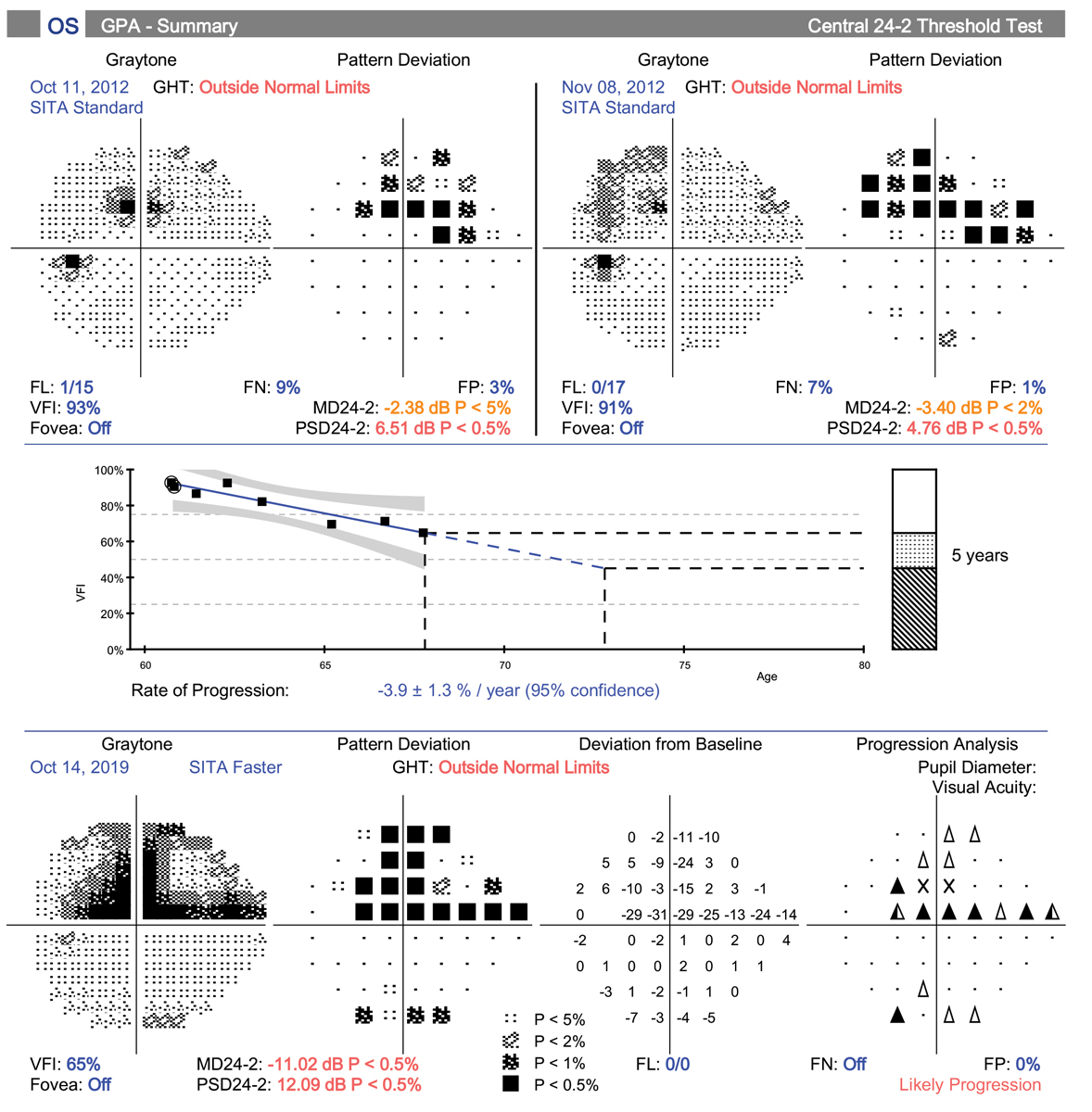 |
| This patient demonstrates rapid progression in the left eye. One of the main reasons is her inability to remain compliant with topical medications. She is also fearful of surgery and has declined several options. She is being considered for a drug delivery system such as Allergan’s Durysta. Click image to enlarge. |
3. Sustained-release drug delivery eases compliance burden.
Many new and exciting sustained-release drug delivery systems are in the pipeline for the treatment of glaucoma, ranging from intracameral implants (Travoprost XR/ENV515, Envisia) to punctal plugs (OTX-TP travoprost insert, Ocular Theraputix) to scleral implants (iDose, Glaukos). However, only one has achieved FDA approval at this time. Durysta (bimatoprost implant 10mcg, Allergan), a biodegradable intracameral implant, gained FDA approval in March 2020.16
Durysta is a sustained-release drug delivery system injected through a clear corneal incision into the anterior chamber and rests in the inferior chamber angle. It slowly releases bimatoprost and dissolves over time. Durysta’s efficacy is comparable to topical bimatoprost, with an IOP-lowering effect that lasts up to six months.17 The FDA approval is based on results from the two 20-month Phase III ARTEMIS studies evaluating safety and efficacy in 1,122 subjects vs. twice-daily topical timolol drops in patients with OAG or ocular hypertension. In these studies, Durysta reduced IOP by approximately 30% from baseline over the 12-week primary efficacy period.
Durysta’s side effect profile is similar to topical bimatoprost and other prostaglandin analogs, but causes minimal to no ocular surface irritation due to its presence in the anterior chamber. However, given its physical location, it is contraindicated in patients with Fuchs’ dystrophy, prior corneal or endothelial cell transplant, and in the absence of a posterior lens capsule or posterior capsular tear.18 Considering the well-documented statistics regarding poor patient compliance with topical glaucoma meds, this implant will take the responsibility out of the hands of the patient at least for a period of time, and will likely prove a reliable treatment option going forward.
 |
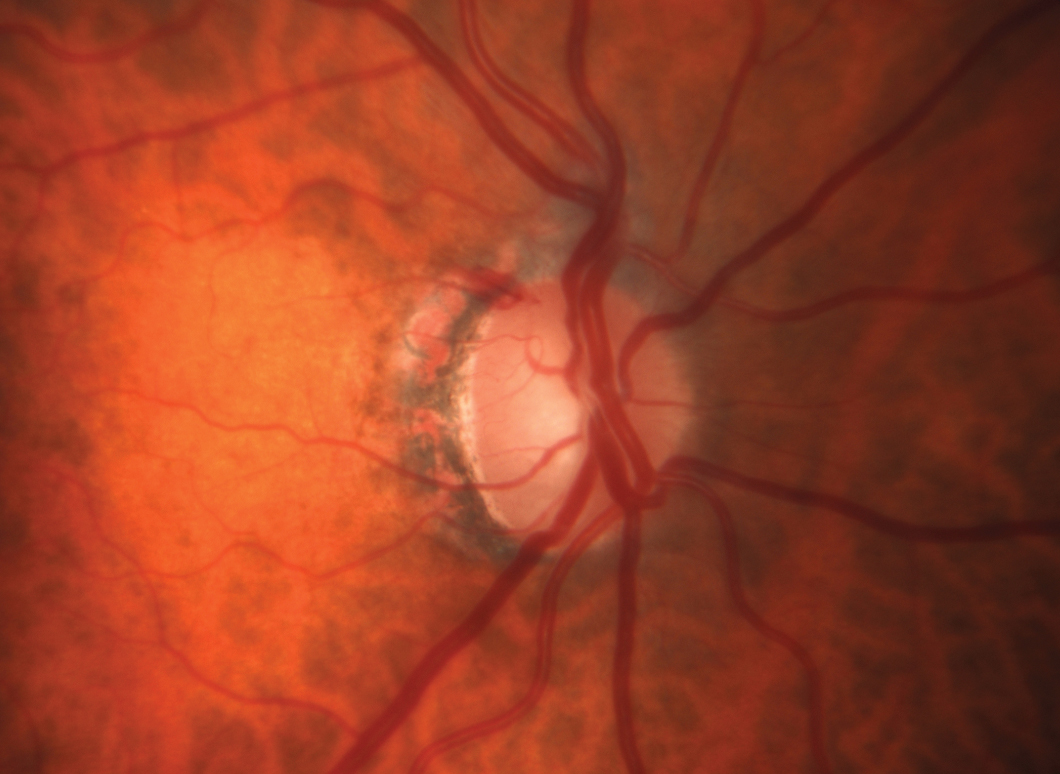
This POAG patient’s pre-treatment IOP was 24mm Hg OD and OS. OCT shows RNFL loss with a clear inferior bundle defect in the right eye. The visual field is just starting to show some abnormal points. After discussing all treatment options with the patient, he elected to have SLT to avoid the topical side effects of medical therapy and the challenges of being compliant. At nine months post-SLT OU his IOP is 17mm Hg OU. Click image to enlarge. |
4. SLT gets the green LiGHT.
SLT has long been recognized as an effective treatment for IOP lowering in mild to moderate glaucoma patients, since its original approval in 2001. SLT’s predecessor, argon laser trabeculoplasty (ALT), has been extensively studied and demonstrated efficacy comparable to medical therapy as an initial treatment for glaucoma.19
ALT and SLT have similar efficacy but, as SLT is less destructive histopathologically, it has the benefit of being able to be repeated.20 However, in the US and other countries, IOP-lowering medication is still the primary treatment offered in most cases for early glaucoma and ocular hypertension.
This conventional wisdom is now in question, and for good reason. In 2019, the results from the Laser in Glaucoma and Ocular Hypertension (LiGHT) study were released, and may lead to a paradigm shift in glaucoma treatment with more patients being offered SLT as an initial treatment option. The results of this observer-masked, randomized controlled trial performed in the UK support the theory that SLT is just as, if not more, effective than medication for maintaining goal IOP.21 In fact, at 36-month follow up, 74.2% of SLT eyes required no drops to maintain goal IOP and were within target at more visits (93%) than in the medication group (91.3%).22 None of the SLT patients required glaucoma surgery to maintain goal IOP during the follow-up period vs. 11 patients in the eye drop group.22
This efficacy, along with a favorable side effect profile and improved cost effectiveness compared with that of topical meds, makes SLT a great choice for first-line therapy.21 It also eliminates the issue of compliance, which is a constant struggle that’s frustrating to ODs everywhere when trying to manage glaucoma with topical medications that are left in the hands of the patient. This pivotal study should influence our decision making going forward when considering initial treatment for glaucoma and ocular hypertension. It forces us to have a conversation with our patients about their initial treatment options, and at the very least to consider referral to a glaucoma specialist capable of performing SLT—including ODs in some states.
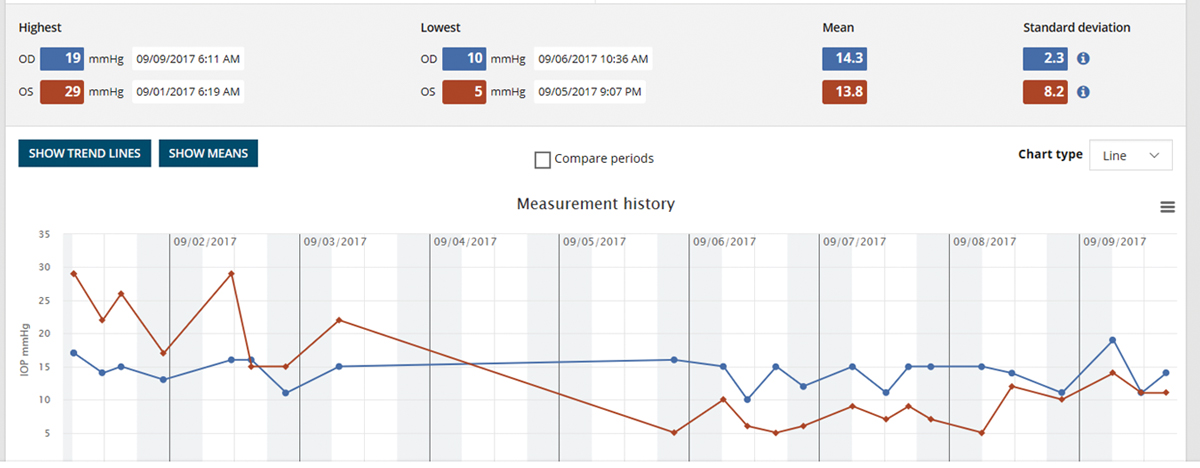 |
The iCare Home measurement report gives the highest and lowest IOP for each eye, as well as the day and time. It provides clinicians a quick overview of IOP measures outside of office hours. Click image to enlarge. |
5. Home-based monitoring reduces dependence on the exam room.
If the last few months have taught us anything, it’s that times are changing. With the onset of COVID-19 and the evolution of how we are living our daily lives in healthcare and beyond, we clinicians may be asked (or forced) to adapt our methods. We are questioning the most safe and sanitary way to check IOP in the office with the debate vacillating between disposable Goldmann applanation tips (Tonosafe, Haag Streit) to Tonopen to iCare. There are also some interesting home care options for glaucoma management now available that may take on a larger role in months and years to come if we find our patients avoiding the office setting due to safety concerns related to the pandemic.
The iCare Home is a “rebound” tonometer that patients can use at home to measure their own IOP, which can be helpful in monitoring the status of their disease and the risk for progression. It is a handheld device with a disposable probe that gently touches the eye (without the need for anesthetic) and takes six rapid measurements. The machine does not display the IOP readings to the patient but rather saves them internally; they are retrieved later on by the eye care provider.
iCare Home has been shown to give a helpful clinical picture of diurnal IOP fluctuations, especially when taken over a seven-day period, and has demonstrated what we think we know already about diurnal flux, with IOP measurements tending to be highest in the early morning and lower later in the day.23 Although it has been accused of correlating poorly with Goldmann applanation tonometry readings, it can be a useful tool to gain the bigger picture in patients that may be progressing despite showing normal readings in the exam room.24 Multiple studies have proven that higher degrees of IOP fluctuation are an independent risk factor for glaucoma progression, and many glaucoma experts agree that the consideration of IOP variability should be a piece of the puzzle when managing glaucoma patients.24 The iCare Home tonometer provides an opportunity to accomplish this feat without having the patient spend 12 hours in the exam room.
Peristat online perimetry, available since 2002, is a free and portable way to screen for field loss outside of the office. The test is available at www.keepyoursight.org and requires nothing more than a computer with a 17” or larger screen.25 The test takes less than five minutes, and results have been shown to correlate well with those of the gold standard 24-2 Humphrey field test.26,27
The Melbourne Rapid Fields (MRF, M&S Technologies) perimetry test has also been shown to correlate well with traditional Humphrey results.28 This program can be used on a tablet or computer screen at home as a web-based exam for glaucoma patients who are concerned about coming into the office during the ongoing COVID-19 crisis. Also, many virtual reality programs offer at-home visual field screening.
In addition to acting as a screening tool for undiagnosed glaucoma patients, the use of these portable and at-home perimetry tests may provide information to us as providers that can help to supplement results from more traditional testing methods in the office.25 They can also act an opportunity for the patient to “practice,” and thereby improve accuracy of field testing in the office at future visits. We learned from the OHTS study years ago that the best accuracy of field test results comes after three or more tests.29
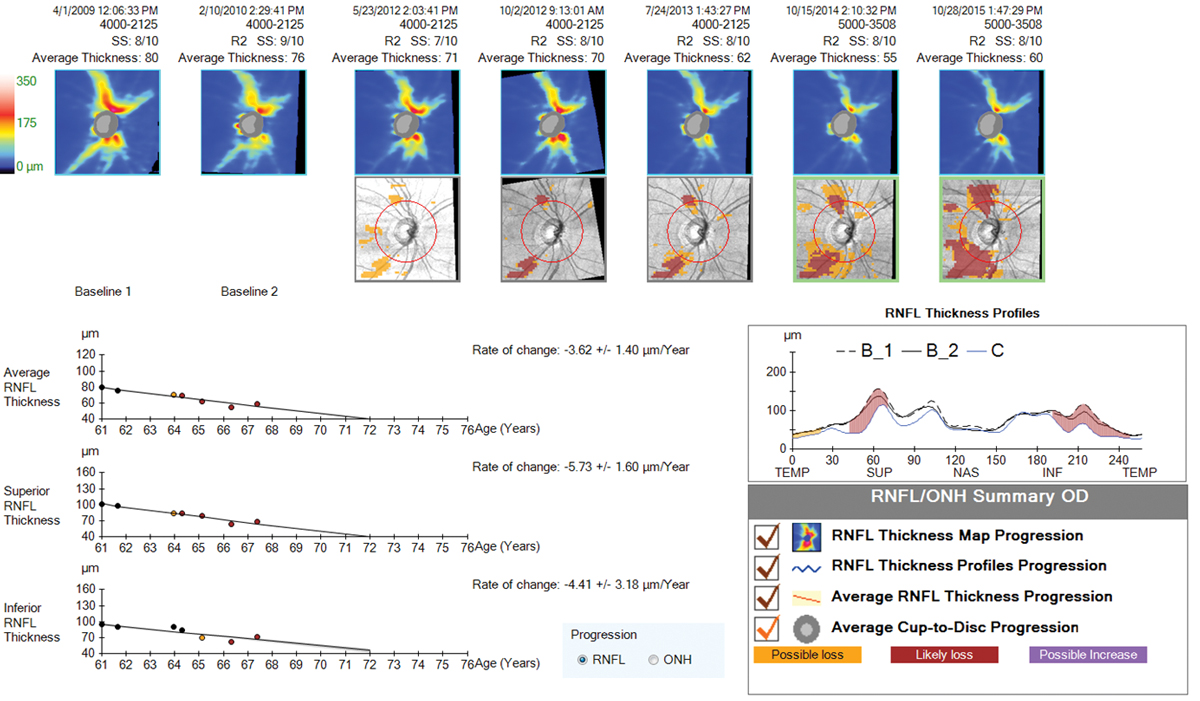 |
| Progression analysis showing significant RNFL loss to the superior and inferior temporal regions over a six-year time period. Despite aggressive treatment approaches, this patient continued to progress with IOP in the low teens. Click image to enlarge. |
6. OCT allows earlier detection of progression.
OCT has been available for nearly 20 years now, with the newer generation (spectral domain) models becoming widely available in the past 10 years. While this technology is amazingly helpful in diagnosing early glaucoma in a typical suspect with apparent optic nerve cupping on exam, we’ve now had the technology for long enough to be able to use it to detect progression of glaucoma as well.
One of the most important measures to look for when trying to detect progression of glaucoma on OCT is repeatable, significant RNFL loss, at both the nerve head and the macular ganglion cell complex (GCC). But what constitutes “significant”? Most experts agree that normal aging accounts for less than one micron per year of average RNFL loss on OCT.30,31 The machine itself has a test-retest variability of about five microns; in light of that, experts agree that about 10 microns (two standard deviations of the machines inter-test variability) of repeatable change on a reliable test would constitute progression.30 A reliable test has a signal strength of 7/10 or better, which is easier to achieve on a dilated pupil, and is most accurate when performed in the same state each time (dilated vs. undilated).
OCT is generally recommended once per year on a glaucoma suspect or mild glaucoma patient who has not shown progression, but is valuable to do more often when progression is suspected, to either confirm past change or look for more.30
Progression confirmed on OCT alone or with a concurrent new field defect should emphasize the need for additional treatment measures and lower target IOP. In advanced glaucoma, clinicians need to beware of the “floor effect,” which occurs when OCT technology ceases to detect further change in RNFL thickness at the nerve head, which occurs when readings approach 40-50 microns.30,31 In this case, RNFL OCT at the nerve is no longer helpful. Macular thickness, however, is still valuable, as it will continue to show decline in late-stage disease.30 Visual fields are crucial in advanced disease as well, as vision loss can and will continue to occur with progression, despite RNFL thickness readings becoming stagnant.
Obtaining an OCT of both the RNFL and GCC at the macula is crucial early on in the diagnosis of glaucoma. In addition to abnormal scans being predictive of future visual field loss and progression, these early tests can be used for comparison to future scans for many years to come in the attempt to catch progression early and to modify therapy as needed.32
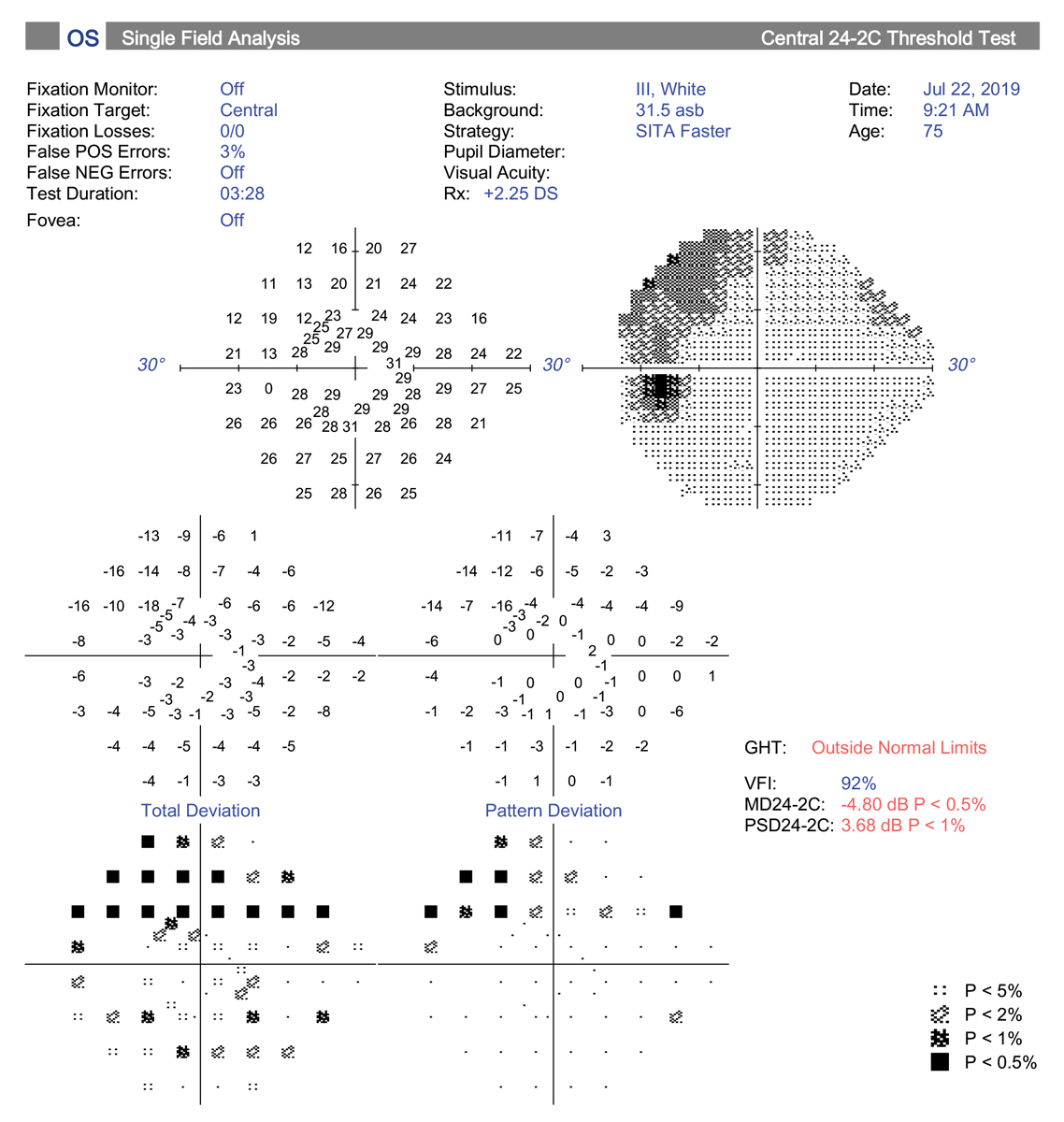 |
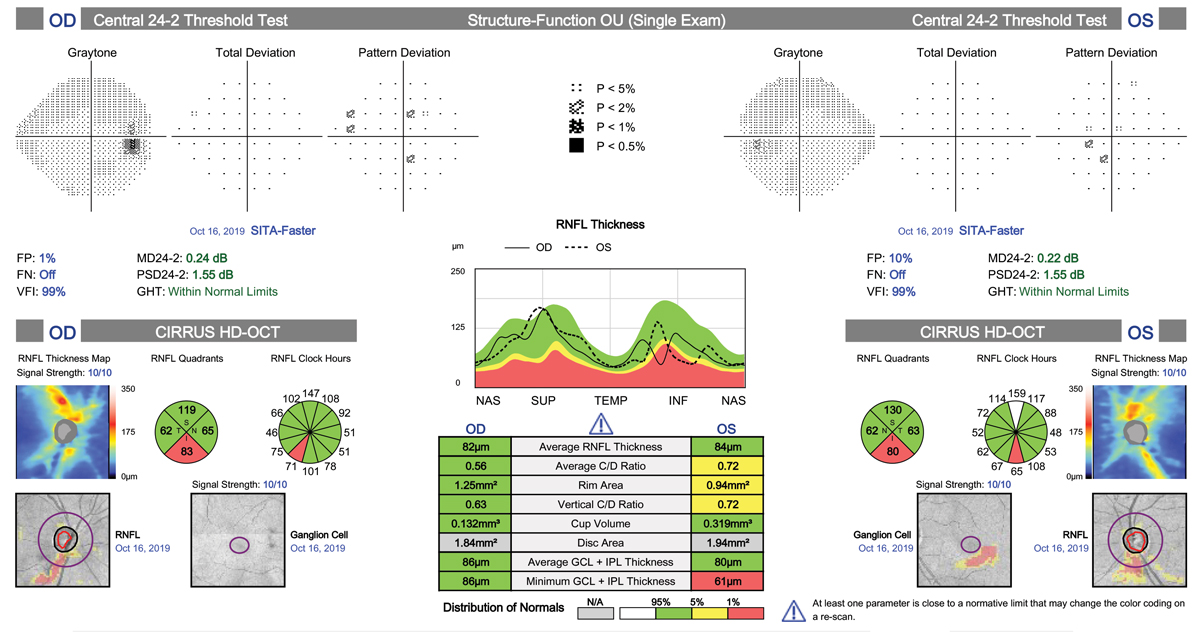
| The central 24-2C test pattern incorporates the new SITA-Faster testing strategy along with 10 extra test locations to the traditional 24-2 grid pattern. This has the potential to replace the 10-2 test for central field testing. Click image to enlarge. |
7. Better visual field testing protocols yield new clues.
In recent years, there have been several important advances in visual field testing for glaucoma. One big change has been increased use of the 10-2 visual field strategy for detecting central defects. One study found central field defects were missed in nearly 40% of glaucoma suspects and 35% of presumed OHTN patients on 24-2 SITA Standard (SS) testing but revealed with the 10-2 strategy.32
This is an important finding, as central field loss leads to decline in vision-related quality of life, decreased central acuity and is predictive of risk for future field progression, especially in patients with normal tension glaucoma.33 Current thinking suggests 10-2 testing should be considered at baseline for all glaucoma suspects and those with diagnosed glaucoma, along with 24-2, to improve detection of small, central defects. The 10-2 pattern is also indicated in cases where OCT macular scan (GCA, GCC) shows loss or thinning.34
Another new advance is development of the SITA Faster test strategy for the Humphrey 24-2. SITA Fast has been around for many years, as long as SITA Standard (SS); they were both developed in 1990s to replace older, slower full threshold test modalities.35 They were found to save time and be more accurate. SITA Faster, which has a duration 30% shorter than SITA Fast and 53% shorter than SITA Standard, has been available since 2019.35 So far, it has proved to be nearly identical to SITA Fast in accuracy and comparability to SS; this is a good thing, as prior studies found that there was no significant difference in the ability to detect glaucomatous field progression between the two test strategies, and only a slight increase in precision of defects with SS.36
One downside to the faster test has been a higher false positive rate, which can lead to unreliable results.37 The advantage lies in having a shorter option for patients who have tended to tire easily or fall asleep on past tests, or those who may have trouble sitting for longer periods due to physical limitations. Another advantage is the ability to perform more frequent VF tests, which will help provide better progression analysis. Most of us have many patients that traditionally “hate” perimetry, and a shorter test duration could certainly attempt to change that mindset. It also helps us as providers to keep things moving in a busy clinical setting without sacrificing quality patient care.
Lastly, in the spirit of combining both the need for central testing and the benefit of increased speed, Zeiss now offers a software package that includes the “SITA Faster 24-2C.” The 24-2C test pattern combines all 24-2 points plus 10 points from the 10-2 strategy centrally, and theoretically could provide the information from the two separate tests into one. This may be an excellent clinical choice moving forward to save time but provide important information regarding peripheral and central visual function in glaucoma patients.
In any given patient, glaucoma usually progresses slowly, giving us ample time to consider our options. But the research supporting our care protocols moves quickly, and it’s incumbent on all of us to keep up with the advances to ensure we do the best job possible in limiting glaucoma’s impact in every affected patient we see.
Dr. Chaglasian is an associate professor at Illinois College of Optometry and chief of staff of the Illinois Eye Institute in Chicago. He is also the current president of the Optometric Glaucoma Society.
Dr. Klein is a fellow of the American Academy of Optometry and a Diplomate of the American Board of Optometry. She is Chief of Optometry at the Flaum Eye Institute of the University of Rochester Medical Center in Rochester, NY.
1. Jalkiewicz JF. Glaucoma treatment takes teamwork. Ophthalmology Management. March 2020:44-46. 2. Kim WI. Combining Minimally Invasive Glaucoma Surgeries. Glaucoma Physician. December 2019:16-19. 3. Fingeret MA. Improved approaches to MIGS for better patient outcomes, Review Education Group. March 5, 2018. www.revieweducationgroup.com/ce/improved-approaches-to-migs-for-better-patient-outcomes. 4. Guedes R, Gravina DM, Lake JC, Guedes V, Chaoubah A. One-Year Comparative Evaluation of iStent or iStent inject Implantation Combined with Cataract Surgery in a Single Center. Advances in Therapy. 2019;36(10):2797–2810. 5. Berdahl J. iStent inject Versus iStent: The iStent inject Advantage. CRST. crstoday.com/articles/maximize-efficacy-minimize-concerns/istent-inject-versus-istent-the-istent-inject-advantage/ 6. Samuelson TW, et al. Prospective, randomized, controlled pivotal trial of an ab Interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract. Ophthalmology. 2019;126:811-821 7. Malvankar-Mehta MS, Iordanous Y, Chen YN, Wang WW, Patel SS, Costella J, Hutnik CM. iStent with Phacoemulsification versus Phacoemulsification Alone for Patients with Glaucoma and Cataract: A Meta-Analysis. PloS one. 2015;10(7):e0131770. 8. Singh IP. Keys to success with the iStent inject, Glaucoma Today. Jan/Feb 2020:35-37. 9. Samuelson et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON study. Ophthalmology 2019;126:29-37. 10. Samples JR. The glaucoma therapy pipeline. Glaucoma Physician, March 2020:20-25. 11. Asrani S, Bacharach J, Holland E, McKee H, Sheng H, Lewis RA, Kopczynski CC, Heah T. Fixed-dose combination of netarsudil and latanoprost in ocular hypertension and open-angle glaucoma: pooled efficacy/safety analysis of phase 3 MERCURY-1 and -2. Advances in Therapy 2020;37(4):1620–1631. 12. Kaufman PL. Latanoprostene bunod ophthalmic solution 0.024% for IOP lowering in glaucoma and ocular hypertension, Expert Opinion on Pharmacotherapy. 2017;18(4):4433-444. 13. Weinreb RN, Sforzolini BS, Vittitow J, Liebmann J. Latanoprostene bunod 0.024% versus timolol maleate 0.5% in subjects with open-angle glaucoma or ocular hypertension: the APOLLO study. Ophthalmology. 2016;123(5):965-973. 14. Medeiros FA, Martin KR, Peace J, et al. Comparison of latanoprostene bunod 0.024% and timolol maleate 0.5% in open-angle glaucoma or ocular hypertension: the LUNAR study. Am J Ophthalmol. 2016;168:250-259. 15. Weinreb RN, Ong T, Scassellati SB, Vittitow JL, Singh K, Kaufman PL. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. Br J Ophthalmol. 2015;99(6):738-745. 16. Durysta FAQ, Allergan. www.durystahcp.com/#faq 17. Lewis RA, et al. Bimatoprost sustained-release implants for glaucoma therapy: 6-month results from a phase I/II clinical trial. Am J Ophthalm. 2017;175:137-147. 18. Durysta prescribing information, Allergan. https://media.allergan.com/products/durysta_pi.pdf. 19. The Glaucoma Laser Trial Research Group. Results of argon laser trabeculoplasty versus topical medicines. Ophthalmology : Journal of the American Academy of Ophthalmology, 1990;97(11), 1403–1413. 20. eyewiki.aao.org/Laser_Trabeculoplasty:_ALT_vs_SLT 21. Dewundara SD. SLT earns a place as first-line therapy. Glaucoma Physician. March 2020:16-18. 22. Gazzard G. et al: Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicenter, randomised controlled trial. Lancet. 2019;393:1505-16. 23. Huang J. et al. Diurnal intraocular pressure fluctuations with self-tonometry in glaucoma patients and suspects: a clinical trial. Optom Vis Sci. 2018;5(2):88-95. 24. Asrani S. Diurnal IOP control: how important is it? Glaucoma Today. July/Aug 2014:36-7. 25. Lowry EA, Ianchulev S, Han Y. Perimetry comes online. Glaucoma Today. July/Aug 2017:40-41. 26. Lowry EA, Hou J, Hennein L, et al. Comparison of peristat online perimetry with the humphrey perimetry in a clinic-based setting. Transl Vis Sci Technol. 2016;5(4):4. 27. Ianchulev T, Pham P, Makarov V, Francis B, Minckler D. Peristat: A computer-based perimetry self-test for cost-effective population screening of glaucoma, Current Eye Research. 2005;30(1):1-6. 28. Xiang Y, Kong G, He M, Crowston JG, Vingrys AJ. A comparison of perimetric results from a tablet perimeter and humphrey field analyzer in glaucoma patients. Trans. Vis. Sci. Tech. 2016;5(6):2. 29. Keltner JL, Johnson CA, Quigg JM, et al. Confirmation of visual field abnormalities in the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2000;118(9):1187-1194. 30. Kent C. Using tech to track glaucoma progression. Review of Ophthalmology. Dec 2017:30-40. 31. Saunders LJ, et al: What rates of glaucoma progression are clinically significant? Expert Rev Ophthalmol. 2016;11(3):227–234. 32. De Moraes CG et al. 24-2 visual fields miss central defects shown on 10-2 tests in glaucoma suspects, ocular hypertensives, and early glaucoma. Ophthalmology. 2017;124:1449-1456. 33. Puspha R. et al. Baseline central visual field defect as a risk factor for NTG progression: a 5-Year prospective study. J Glaucoma. 2019;28:952–957. 34. Hood D, De Moraes CG. Four questions for every clinician diagnosing and monitoring glaucoma. J Glaucoma. 2018;27:657–664. 35. Heijl A, et al. A new SITA perimetric threshold testing algorithm: construction and a multicenter clinical study. Am J Ophthal. 2019:198:154-165. 36. Saunders L, et al. Measurement precision in a series of visual fields acquired by the standard and fast versions of SITA analysis of large-scale data from clinics. JAMA Ophthalmol. 2015;133(1):74-80. 37. Phu J, et al. Clinical evaluation of SITA–faster compared with SITA–standard in normal subjects, glaucoma suspects, and patients with glaucoma. Am J Ophthal. 2019; 208: 251-264. |

