
A 54-year-old white female was seen in consultation for a comprehensive ophthalmic evaluation. Her medical history was significant for breast carcinoma (in remission) and hypertension. She was currently medicated with tamoxifen, losartan potassium and aspirin. She reported no pertinent ocular history, social history or drug allergies. Her family history was remarkable for an autosomal dominant inheritance disorder characterized by migraine headache and stroke.
A month prior to her visit, a positron emission tomography (PET) scan revealed multiple lung lesions. Initially read as metastatic lung carcinoma, she was referred to a pulmonologist for pulmonary function tests (PFTs) and excisional biopsy. PFTs were unremarkable, but a bronchoscopy revealed multiple granulomas. She was subsequently sent for ophthalmic assessment.
Her best-corrected visual acuities were 20/30 and 20/40 in her right and left eyes, respectively. Ocular motility was full with no limitation and pupils were equal, round and reactive to light with no afferent defect. Confrontation fields were full to finger counting. Ocular alignment was normal with an orthophoric position in primary and lateral gazes. Additionally, she noted 7/7 color plates in each eye. Intraocular pressure measured 16mm Hg in each eye. The anterior segment was unremarkable. Fundus exam showed healthy lenticular, vitreous, vascular and chorioretinal structures. The optic nerves showed a cup-to-disc ratio of 0.3 with minimal temporal disc pallor in both eyes.
| Fig. 1. FLAIR image showing periventricular white matter lesions. |
Although she was asymptomatic, both eyes exhibited a loss of best-corrected visual acuity, which raised a red flag. Subsequently, we ordered spectral-domain optical coherence tomography (SD-OCT) and visual field testing. The SD-OCT showed mild temporal retinal nerve fiber layer (RNFL) thinning with corresponding nasal visual field defects. Additional cranial nerve testing revealed partial left facial nerve palsy. In light of her history and clinical findings, our differential diagnosis included inflammatory, demyelinating and neoplastic causes. Subsequently, we ordered magnetic resonance imaging (MRI) of the head and neck with and without contrast and fat suppression.
Intracranial imaging revealed multiple periventricular white matter (PWM) lesions. Causes of PWM lesions are extensive but most commonly include normal senescent changes, hypertension, focal cerebrovascular accidents, demyelination, migraine, vitamin B6 deficiency and infectious or inflammatory-related vasculitis, including but not limited to Lyme disease and sarcoidosis.1 Additional imaging of the neck revealed swelling of the jugulodigastric and mediastinal lymph nodes. The etiologies of enlarged lymph nodes are numerous but most commonly include infection, neoplasm and inflammation.
In this case, the findings of lung granulomas, bilateral optic atrophy, facial nerve paresis, enlarged mediastinal lymph nodes and PWM lesions led us to a presumptive diagnosis of neuro-sarcoidosis; she was subsequently referred to a neurologist who confirmed the diagnosis.
Discussion
Sarcoidosis is a multi-organ disease resulting from increased cellular immunity of unknown etiology. Activation of T-cell and B-cell lymphocytes results in the production of granulomas and immunoglobulins. The incidence of sarcoidosis in the United States has been reported to range from 0.01 to 0.04%. It typically affects adults within the second to fourth decades of life, and is more frequently found in blacks and females.2 While the exact mechanism of sarcoidosis is unknown, genetic factors and infectious agents exist that increase susceptibility to developing the disease.3
Neuro-sarcoidosis is most often a sequela of systemic sarcoidosis. It primarily affects the meninges, intracranial blood vessels and spinal cord. While its incidence is only reported in 5% of living patients, a postmortem series discovered central nervous system (CNS) involvement in as many as 25% of cases.4 In rare instances, neuro-sarcoidosis can present as isolated central nervous system disease. These cases are much more difficult to definitively diagnose since a biopsy may not be possible or desirable due to the anatomic location of the granuloma.
Seventh nerve palsy is the most common focal manifestation of neuro-sarcoidosis, occurring in up to 50% of patients, by optic and vestibular nerve involvement.5 In most cases, the clinical presentation of neuro-sarcoidosis is nonspecific, with symptoms that include weakness, paresis, paresthesia, diplopia and dysarthria. In the absence of confirmed systemic granulomas, demyelinating diseases such as multiple sclerosis (MS) and chronic inflammatory demyelinating polyneuropathy may be easily confused.
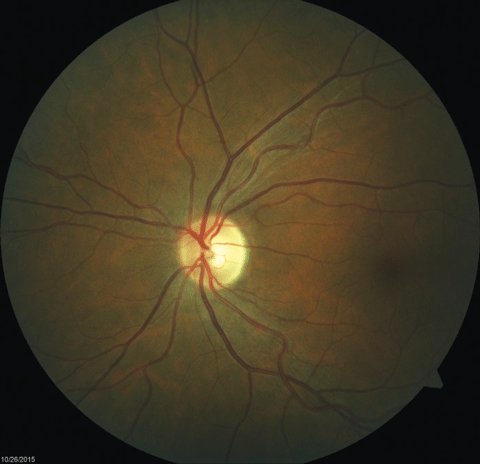 | 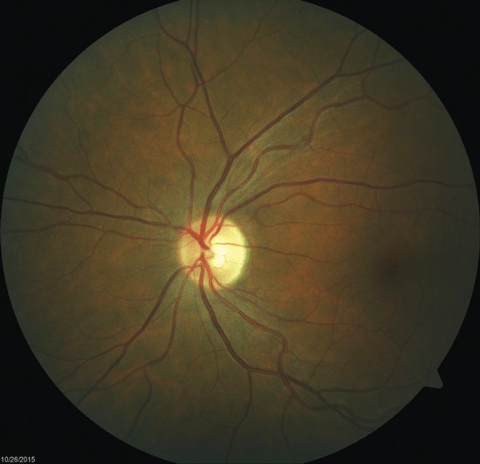 |
| Fig. 2. Fundus images of the patient’s left and right eyes, respectively, which revealed mild bitemporal optic nerve pallor. Click images to enlarge. | |
Imaging of the brain, orbits and spinal cord are invaluable in making a diagnosis of neuro-sarcoidosis since central nervous system involvement may affect the parenchyma, nerve roots, leptomeninges, dura mater, surrounding bone and optic nerve. The most typical neuroimaging finding of neuro-sarcoidosis is enhancement of the leptomeninges; however, this is only present in up to 40% of cases with central nervous system disease.6 More commonly, neuroimaging of the brain will show nonspecific intraparenchymal abnormalities described as non-enhancing, multiple white matter lesions seen as hyperintense signals on T2-weighted imaging. Often, a diagnosis of probable neuro-sarcoidosis can be considered without confirmed biopsy in the presence of contrast enhancement of the leptomeninges and anterior visual pathway, facial nerve paresis and response to an empiric trial of high-dose corticosteroids.
Understanding Neuro-ophthalmic Sarcoidosis
Neuro-ophthalmic manifestations of sarcoidosis represent a subtype of neuro-sarcoidosis afflicting up to 15% of patients with systemic disease.7 The most common neuro-ophthalmic findings include optic neuritis, ocular motility disorders, chiasmal involvement and lesions of the optic tract. Patients presenting with any of the above-mentioned neuro-ophthalmic signs and symptoms should initially undergo MRI of the brain and orbits with and without contrast and fat suppression.
Findings consistent with enhancement of the lacrimal gland, meninges, hypothalamus or pituitary stalk are high-risk signs of central nervous system disease. These patients should be evaluated for systemic involvement with chest radiography, serum angiotensin-converting enzyme (ACE), anergy panel, Quantiferon gold tuberculosis test (Cellestis, Australia), 24-hour urine calcium and lumbar puncture.8 Additional testing through a whole-body gallium scan can also show uptake related to both systemic and central nervous disease but is not a primary test since it lacks specificity.
In all forms of neuro-sarcoidosis, the first line of treatment is systemic corticosteroids dosed at a range of 40mg to 80mg per day, with approximately 50% of patients showing significant improvement.9 Alternatively, low-dose cyclosporine can be used as a safe and effective treatment in patients who are intolerant to corticosteroids.10 Other immunomodulary therapies for neuro-sarcoidosis include azathioprine, cyclophosphamide, chlorambucil and methotrexate.11
In our patient, the clinical findings of lung granulomas, facial nerve paresis and optic nerve atrophy prompted urgent MRI of the brain and orbits with and without contrast and fat suppression to discount active central nervous system involvement.
While no evidence of enhancing intracranial lesions or granulomas existed, the presence of PWM lesions in a younger patient without small vessel disease made us suspicious for intraparenchymal inflammation.
Integrating Care
Neuro-sarcoidosis is a rare disease entity, but should be suspected in patients with isolated central nervous system symptoms, including optic atrophy and facial nerve palsy as well as cases of systemic sarcoidosis that present with neuro-ophthalmic findings.
Management of the condition should include urgent neuroimaging and referral to a subspecialist trained in the sufficient management of central nervous system disease. Ophthalmic follow-up should include serial testing of the visual fields and optic nerve with both optical coherence and fundus photography.
Be sure to keep open the lines of communication between the primary eye provider, pulmonologist and neurologist. In these cases, sufficient communication between specialties is neccesary for integrated care and appropriate management of your patients condition, ensuring that they receive an optimized outcome and quality of life.
|
1. Barkhof F, Scheltens P. Imaging of white matter lesions. Cerebrovasc Dis. 2002;13(Suppl 2):21-30. 2. Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336:1224–34. 3. Obenauf CD, Shaw HE, Sydnor CF, et al. Sarcoidosis and its ophthalmic manifestations. Am j ophthalmol. 1978;86:648-55. 4. Romero JI, Diaz-Carasco DM. Ataxia como primera manifestacion de neurosarcoidosis. Neurologia Argentina 8, 68-70. [Cross Reference] 5. Stern BJ, Krumholz A, Johns C, et al. Sarcoidosis and its neurological manifestations. Arch Neurol 1985;42:909-17. 6. Smith JK, Matheus MG, Castillo M. Imaging manifestations of neurosarcoidosis. AJR. 2004;182:289-95. 7. Jarnier D, Series C. Neurosarcoidosis. Review of the literature. Neurochirurgie. 1999;45:214-8. [Cross Reference] 8. Frohman LP, Grigorian R, Bielory L. Neuro-ophthalmic manifestations of sarcoidosis: clinical spectrum, evaluation, and management. Journal of Neuroophthalmology. 2001;21(2):132-7. 9. Delaney P. Neurologic manifestations in sarcoidosis: review of the literature, with a report of 23 cases. Ann. Intern. Med. 1977;87(3):336–45. 10. Bielory L, Frohman L. Low-dose cyclosporin therapy of granulomatous optic neuropathy and orbitopathy. Ophthalmology. 1991;98:1732-6. 11. Agbou B, Stern B, Sewell C, et al. Therapeutic considerations in patients with refractory neurosarcoidosis. Arch neurol. 1995;52:875-9. |

