 A 68-year-old white female, who relocated to my area, was referred to my office for continuation of her glaucoma care. According to her records, which her previous provider sent to me, she had been diagnosed about three years earlier with open-angle glaucoma in both eyes.
A 68-year-old white female, who relocated to my area, was referred to my office for continuation of her glaucoma care. According to her records, which her previous provider sent to me, she had been diagnosed about three years earlier with open-angle glaucoma in both eyes.
Her records also reported that her pre-treatment IOP averaged 24mm Hg OD and 26mm Hg OS. Pachymetry readings were 531µm OD and 527µm OS. Visual fields with standard automated perimetry were clear OU with no discernible defects. Her cup-to-disc ratios were estimated to be 0.50 x 0.60 OD and 0.60 x 0.60 OS. The previous provider prescribed Lumigan (bimatoprost 0.01%, Allergan) one drop OU HS. Subsequently, the patient’s post-treatment IOP averaged 17mm Hg OU.
Diagnostic Data
When I first saw the patient in early July 2013, her best-corrected visual acuity was 20/20 OD and OS. Pupils were equal, round and reactive to light and accommodation with no afferent defect. Her other medications included Celexa (citalopram, Forest Laboratories) and lisinopril; she reported no allergies to medications.Slit lamp examination of her anterior segments was unremarkable except for a mild to moderate amount of guttatae in both corneas, but no evidence of striate keratopathy. Pachymetry measured 530µm OD and 522µm OS. Intraocular pressure measured 16mm Hg OD and 17mm Hg OS at 10:30 AM—consistent with her previous IOP measurements. Gonioscopy revealed 3+ open angles in both eyes, with ciliary body visible in several areas, and grade 1 trabecular pigmentation OU.Upon dilation, both crystalline lenses were unremarkable, other than for age-consistent incipient nuclear sclerosis. There were complete posterior vitreous separations present OU. Cup-to-disc ratios appeared to be 0.70 x 0.75 OD and 0.70 x 0.80 OS through stereoscopic evaluation at the slit lamp. Her retinal vasculature was normal in both eyes. Both macular and peripheral retina evaluations were largely unremarkable. There were no holes, tears or tractional phenomenon in either eye.
At this visit, I obtained Heidelberg Retina Tomograph-3 (HRT-3, Heidelberg Engineering) baseline images, as well as stereo photos of both optic nerves. HRT-3 images correlated well with my subjective estimation of the cup-to-disc ratio in both eyes. The inferotemporal sector of the neuroretinal rim in each eye was outside of the statistical norms of the Moorfields Regression Analysis (MRA). This corroborated the appearance of the neuroretinal rims OU, as the inferotemporal aspects were slightly thinned and no frank notching was present. The clinical evaluation of the optic nerves showed that they did not follow the ISNT (inferior-superior-nasal-temporal) rule, and this too was confirmed by HRT-3 imaging. In short, all of the measurements at this patient’s initial visit with me were essentially the same as those from her previous provider—except for one surprising thing: her cup-to-disc ratios were markedly different.
Could she have progressed in the eight months since her last visit with her other optometrist? Or, are we simply looking at inter-observer differences?
Follow-Up
I attributed the disparity in the cup-to-disc ratios to inter-observer differences. Accordingly, I asked the patient to continue her Lumigan HS OU, and to see me again in two months.The patient returned as directed in early September 2013. IOP was 16mm Hg OU at 8:45 am. Threshold visual fields on Heidelberg Edge Perimeter (HEP) demonstrated an early superior arcuate defect in each eye. OCT imaging showed mild thinning in the inferotemporal retinal nerve fiber layer (RNFL) in both eyes—in the same sectors that were aberrant on the MRA obtained at the last visit. While the visual field defects were new findings, the testing strategies of her first field with me and the previous fields with her former provider were different (SAP vs. HEP). The HEP-identified defects were mild and, from a practical perspective, may simply have been shallow enough not to be picked up on SAP.In other words, I did not consider these definable field defects as a worsening of her condition, but rather a finding from a different field instrument. I looked closely at her optic nerves, and once again I thought that her cup-to-disc ratios appeared larger than her previous provider had reported. DiscussionThis case illustrates something we see quite regularly: the effects of inter-observer differences. In glaucoma, the one major subjective element supplied by the provider is the cup-to-disc ratio.
 |
|
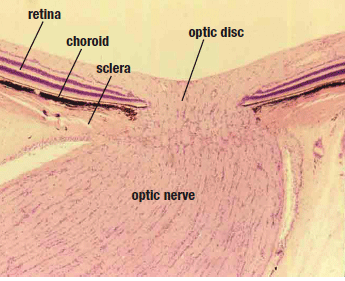
| 1. Histological section of the optic nerve. Bruch's membrane is very
visible and all retinal ganglion cells pass through its opening. |
|
|
|
|
 |
|
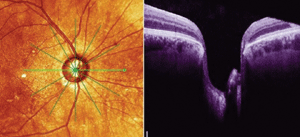
| 2. Cross-sectional OCT scan of a different patient with advanced
glaucomatous disease. Note the extremely thinned neuroretinal rim adjacent to Bruch's membrane. |
Further, be aware that all ganglion cells, as they are heading posteriorly through the optic nerve head, do so medially to Bruch’s membrane. In other words, no retinal ganglion cells travel lateral to the edge of Bruch’s membrane. While Bruch’s membrane is not visible at the slit lamp, it clearly is visible on OCT imaging. And because all retinal ganglion cells traverse the optic nerve through Bruch’s membrane opening (BMO), several authors suggest that BMO should be the objectively definable lateral aspect of the optic disc. To that end, researchers are investigating the influence of OCT-visible optic disc margins on neuroretinal rim evaluations.
They’re finding that what we see clinically, and what we define at the slit lamp as the optic disc margin, is very different from that canal bordered by BMO as seen with OCT. In other words, what we call the “disc” is, in many respects, not what the anatomical disc appears to be. In the image below, we can see how the scleral opening is much wider than the Bruch’s membrane opening; so depending on what we use to define the disc margin—BMO or scleral canal—has a dramatic effect on the cup-to-disc ratio.
In the OCT scan of a different patient (Figure 2), note the clearly defined medial edge of Bruch’s membrane, and note how thin the neuroretinal rim is adjacent to Bruch’s membrane. If we define the BMO as the lateral aspect of the optic disc on OCT, measurements can then be made of the adjacent neuroretinal rim tissue, and monitored over time.What does this mean? Quite simply, uniformity—in how optic nerves are measured by an objective instrument such as an OCT. This uniformity would reduce inter-observer variances and, perhaps one day soon, permit us to define the optic nerve without using the term “cup-to-disc ratio.”

