 |
A 56-year-old black woman presented for a comprehensive ocular examination. Prior to a relocation, her long-time ophthalmologist had been treating her for glaucoma. The patient reported using latanoprost 0.005% QHS OU, dorzolamide/timolol maleate fixed combination BID OU and brimonidine 0.2% solution TID OU. She further reported having had laser treatment for glaucoma in both eyes (most likely laser trabeculoplasty) within the past two years.
Examination
Her best-corrected visual acuity was 20/20 OD and 20/40-1 OS. Confrontation visual fields were full in the right eye but severely constricted in the left. Consistently, the left eye displayed a 3+ relative afferent defect on pupil testing. Intraocular pressure (IOP) was measured at 20mm Hg OD and 28mm Hg OS. Gonioscopy revealed open angles to the ciliary body in all four quadrants, with moderate pigment and no sign of angle recession in either eye. Central corneal thickness measured 517µm in the right eye and 504µm in left. Upon dilated examination, the optic nerves were markedly cupped, measuring approximately 0.8/0.8 OD and 0.9/0.95 OS. OCT evaluation showed notable retinal nerve fiber layer (RNFL) damage superiorly in the right eye and extensive RNFL damage both superiorly and inferiorly in the left eye. Since the patient was already on maximum tolerable medications and reported excellent compliance, we ordered a consultation with a glaucoma specialist. After examining her and reviewing the testing, he recommended proceeding with transscleral cyclophotocoagulation (TS-CPC) in the left eye using the Cyclo G6 system (Iridex).
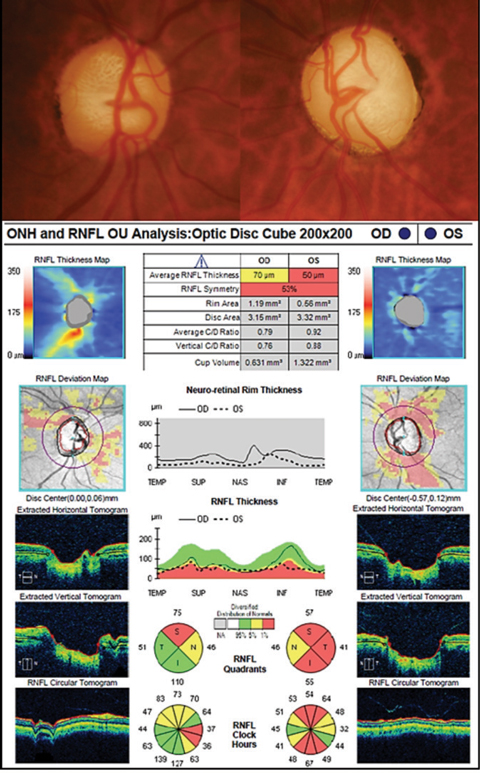 |
| Fundus photos and OCT images of our 56-year-old glaucoma patient. Click image to enlarge. |
Procedural Process
Cyclodestruction procedures aim to decrease IOP by diminishing the ciliary body’s capacity to produce aqueous. The earliest cyclodestructive techniques involved the use of extreme cold (-80° C) applied to the conjunctival surface circumferentially around the cornea, approximately 4mm beyond the limbus; this procedure is referred to as cyclo-cryotherapy.1
Limitations of cyclocryotherapy include a variable response, in which patients may experience no IOP lowering effect or extreme lowering to the point of hypotony.2,3 Moreover, a host of complications may be seen, including intraocular hemorrhage, secondary cataract, retinal detachment and phthisis bulbi, any of which can lead to further loss of vision and even enucleation.2,3 Today, cyclocryotherapy is rarely used, but similar techniques that employ ophthalmic lasers (collectively referred to as cycloablative procedures) remain an option for some glaucoma patients.
Endoscopic cyclophotocoagulation is invasive and uses a laser endoscope to allow direct visualization and treatment of the ciliary processes by means of a semiconductor diode laser.4 The procedure is typically performed in conjunction with cataract surgery for patients with moderate to severe glaucoma.5 TS-CPC is a noninvasive procedure that uses a diode laser attached to a specialized contact probe which is applied to the conjunctival surface around the limbus. Like cyclocryotherapy, TS-CPC has been criticized for its lack of predictability and propensity toward complications, which include hypotony, early postoperative inflammation and pain, cystoid macular edema, persistent flare and loss of vision.6
An Updated Approach
Most glaucoma surgeons today view TS-CPC as an option only for those patients who have failed more invasive surgeries (i.e., trabeculectomy or tube shunt procedures) or those who have minimal or no useful vision, but experience chronic pain associated with uncontrolled IOP.7 The procedure is generally frowned upon for patients with good vision and those who have only been previously managed with topical meds. However, those attitudes appear to be changing, and some have even suggested that this procedure may be a viable first-line surgical treatment.8,9
This potential paradigm shift can be attributed to new technology and a modified treatment approach. The Cyclo G6, when coupled with the MP3 glaucoma probe, employs micropulse technology; this differs substantially from the continuous wave laser that was used in prior versions of TS-CPC. As its name implies, the micropulse platform produces short bursts of laser energy with intervening rest periods, permitting for tissue cooling and rebound.8 This ensures cellular disruption without total cell destruction, and while the mechanism of action is not completely understood, researchers theorize that the thermal insult may activate a cellular biochemical cascade, resulting in an IOP-lowering effect.10
As experts explain, this unique platform offers some distinct advantages over other surgical techniques. Unlike invasive procedures such as trabeculectomy or tube-shunt surgery, micropulse TS-CPC is theoretically repeatable (much like SLT). Moreover, it has the capacity to be performed as an in-office procedure, although some surgeons still recommend it be done in an outpatient facility for greater safety.11 TS-CPC appears to provide significant results that can be tailored to the patient’s needs. Studies show IOP reduction ranging from 30% to 45% among populations with varied types and severity of glaucoma.12-16 They also show a diminished need for multiple medications following treatment.12-16 In one trial, the average number of glaucoma medications fell from 3.3 down to 1.8 after the procedure.16
Micropulse TS-CPC can be associated with a significant amount of discomfort, to the point that it requires a retrobulbar block, often with the adjunctive use of oral anxiolytics or intravenous sedatives.16 Physicians can also anticipate substantial inflammation after the procedure, necessitating the use of strong topical corticosteroids during the immediate postoperative period. Of course, the greatest concern with this technique has been the potential for vision loss, particularly the “snuffing out” of central fields in sighted patients with advanced disease.
In a 2010 study involving 49 glaucomatous eyes with best-corrected visual acuity of 20/60 or better, 18.3% experienced a loss of greater than or equal to two Snellen lines within 12 months of the procedure.6 After five years, 30.6% of the eyes showed a similar loss of vision.6 The authors found this outcome “concerning,” but also concluded that much of the visual deterioration was likely due to the natural course of the disease rather than the intervention itself. They cited a 2007 study in which the same magnitude of vision loss was seen in approximately 33% of eyes undergoing trabeculectomy or tube-shunt surgery after one year of follow-up.17
Advocates of this new technology argue that any surgical intervention places the patient at risk for complications and progression, particularly in advanced glaucoma. But they insist micropulse TS-CPC is more predictable and efficacious in the hands of a well-trained surgeon than laser trabeculoplasty, minimally invasive glaucoma surgery or filtration surgery, while maintaining an acceptable safety profile and the potential for repeatability. Advocates also point to the rising cost of medications, declining insurance coverage and diminishing physician reimbursement for more invasive glaucoma surgeries as a rationale to consider TS-CPC earlier in the disease course.8
1. De Roetth A. Cryosurgery for the treatment of glaucoma. Trans Am Ophthalmol Soc. 1965;63:189-204. |

