 |
History
A 27-year-old Caucasian female reported to the office urgently with a chief complaint of pain and vision changes in her right eye throughout the previous week. She explained that she had scratched her right eye following aggressive rubbing when her seasonal allergies caused irritation during an outing, four weeks earlier. She recounted that she did not seek care at the time of the incident and opted to treat it herself with over-the-counter lubricants.
She believed her remedy was working until about a week prior to the office visit when she noticed her eye was red, feeling scratchy and that her vision “was off.” Her previous ocular and systemic history was unremarkable, she was not a contact lens wearer and she denied allergies of any kind.
Diagnostic Data
Her best uncorrected entering visual acuities were 20/50 OD and 20/20 OS at distance and near with no improvement upon pinhole. Her external examination was normal with no evidence of afferent pupil defect. The biomicroscopic examination of the right eye’s anterior segment is shown in the photo; mild sodium fluorescein corneal staining overlying a 3mm x 1mm area of mild subepithelial and stromal corneal edema with subepitheilal infiltrate, local limbal conjunctival injection and trace cell and flare in the anterior chamber, OD. The fellow eye was normal. Goldmann applanation tonometry measured 13mm Hg OD and 15mm Hg OS. The dilated fundus exam was normal with no posterior pole or peripheral pathologies in either eye.
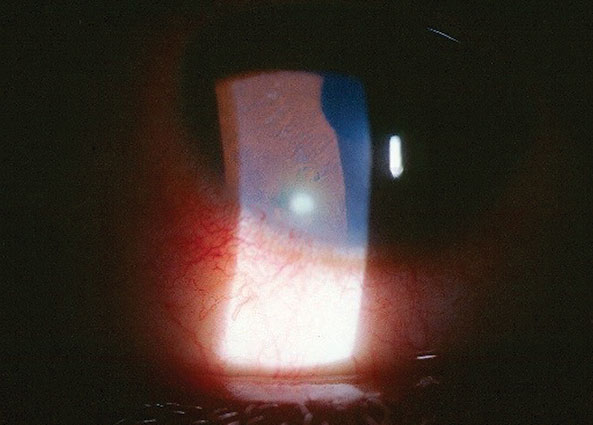 |
| This 27-year-old woman reported pain after scratching her right eye. |
Your Diagnosis
Does the case presented require any additional tests, history or information? What steps would you take to manage this patient? Based on the information provided, what would be your diagnosis? What is the patient’s most likely prognosis?
Discussion
Additional studies of the patient’s right eye included detailed measurement of the lesion, lid eversion to rule out follicles, papillae, pseudomembrane or foreign body, nodes were palpated, lavage was performed to insure a chemically neutral ocular surface, photodocumentation was accomplished and a 2% to 0.5% phenelephrine test was completed to insure the inflammation was superficial, not occurring in deep tissue (rapid blanching). The lids were also inspected to rule out significant concurrent blepharitis or Demodex infestation. Given the history, no laboratory studies were considered necessary at this time.
The diagnosis in this issue is marginal keratitis (MK), OD secondary to an unmanaged mechanical corneal abrasion caused by eye rubbing precipitated by seasonal allergies. Marginal keratitis is defined as a peripheral corneal inflammatory response.1-4 It can be produced following chronic exposure with an adjacent antigen (make up, chemical exposure, microbes), a chronic mechanical stimulus (debris, eyelid or eyelash), induced hypoxia (contact lens related) or as a sequela of vasculitic systemic disease (engraftment syndrome-early complication of hematopoietic stem cell transplantation, leucocytoclastic vasculitis).1-14 Patients with marginal keratitis (MK) may range in presentation from completely asymptomatic to severely symptomatic, depending upon the extent and duration of the reaction. When present, symptoms are graded as mild, moderate and severe and may include ocular discomfort (e.g. burning, foreign body sensation, grittiness), photophobia and chronic tearing.6 Vision normally remains unaffected.1-7
Typically, bulbar conjunctival injection is mild to absent. In the Fuchs’ variation, pseudopterygium with mild corneal thinning may be noted.14 The palpebral conjunctiva may demonstrate subtle conjunctival chemosis.11-13 The key diagnostic sign is one or more focal areas of grayish, subepithelial infiltrate near the limbus, usually located in the inferior cornea.1,2,11-15 The positions where the cornea interacts with the lower eyelid margin are particularly common sites of involvement.1,2,13-15 When the overlying epithelium is compromised, the defect is usually seen as an interrupted stippling much smaller than the area of infiltrate. This is in contradistinction to microbial keratitis which demonstrates a continuous area of epithelial defect virtually equal to the area of stromal infiltrate.16 Characteristically, there are zones of unaffected limbal cornea.9 In rare instances, MK may be accompanied by other inflammatory ocular sequelae, such as mild anterior uveitis or folds in Descemet’s membrane associated with corneal edema. Blepharitis and its variants (seborrhic, psoriasiatic, acne rosacea, demodex) are major contributors secondary to their effects on the lid margin.2,11-13,17-19 Patients with MK often have a history of other ocular surface disease, such as chronic dry eye or allergic conjunctivitis. Inflammatory autoimmune disorders, including Terrien’s marginal degeneration, Steven’s Johnson syndrome, rheumatoid arthritis, Wegener’s granulomatosus, Behçet’s disease and Churg-Strauss syndrome are also known to produce MK.1,2,8-13,15,16,19-21
Classic MK represents as a localized immune response, believed to be driven by antigen-antibody complexes that deposit in the peripheral corneal stroma.11,20-23 Generically, the mechanism is an inflammatory process which initiates a cascade that results in the influx of leukocytes and plasma molecules to the site of the tissue damage.1,24,25 The inciting etiology will dictate the specific cellular response.15,16,25-27 Initially, the overlying epithelium remains intact; however, as inflammatory cells accumulate to neutralize the offending reaction, collagenolytic enzymes released from these cells induce noninfectious ulceration (infiltrate in the presence of an overlying corneal break).15,28 Matrix metaloprotinase-9 (MMP-9) appears to be a prominent player in the initiation of the epithelial basement membrane degradation that precedes corneal ulceration.28
Historically, bacterial exotoxins from Staphylococcal organisms are considered the primary etiology.11,13,15,20,22 While bacterial overgrowth associated with chronic blepharitis remains a significant cause of MK, clearly not all cases are caused by microbial flora.4-9 Other causes include systemic autoimmune disorders, mechanical events and hypersensitivity reactions to foreign substances and topical drugs including phenylephrine, gentamicin, atropine, pilocarpine and dorzolamide.2,3- 11,15,16,26,27 Corneal hypoxia and bacterial biofilm associated with soft contact lens wear represent yet another potential etiology (though in these instances, clinicians tend to use the term contact lens-induced peripheral ulcer (CLPU).5
The treatment strategy for MK must address both extinguishing the inflammatory response and removing or controlling the underlying microbial, mechanical, toxic, hypoxic or autoimmune etiology.2-27 In cases where microbial flora are implicated, aggressive control of eye lid and ocular surface bacteria can be accomplished using topical and oral antibiotics. Mechanical cleaning of the eyelids to soften and remove debris/microbes should be instructed, using warm compresses and commercially available eye lid cleansers such as OCuSOFT or generic “no-more-tears” baby shampoo, two to four times daily.29 Topical application of green tea tree oil and metronidazole ointment bid along with oral ivermectin, dosed once and repeated in seven days if necessary, are indicated in cases of demodex infestation.30 In cases of rosacea (meibomian gland dysfunction), oral tetracycline, doxycycline or azythromicin can be prescribed.31,32 Generic staphylococcal blepharitis can be treated with traditional topical fluoroquinolone antibiotic drops and or ointments QID. Since inflammation is an integral portion of the entity’s histopathology, its mitigation can be accomplished with either topical antibiotic-steroid combination drops or ointments bid-qid or the addition of a topical steroid to the topical antibiotic.2-27 Topical corticosteroid drops and ointments include fluorometholone, prednisolone acetate, lotoprednol etabonate and difluprednate, bid-q3h, depending on the severity of the case.2-27 Since topical steroids may increase intraocular pressure (IOP), any prolonged course should include IOP monitoring. In cases that produce iritis, cycloplegia may be warranted.
MK associated with drug hypersensitivity necessitates discontinuing the noxious agent and controlling inflammation as forementioned.26,27 Cases of CLPU warrant temporarily discontinuing lens wear, protecting the cornea with a topical antibiotic or antibiotic-steroid combination, rehabilitating the ocular surface. Wear can continue following a reevaluation of the fit, lens material and disinfection system.5
Management options associated with systemic autoimmune diseases require a team approach. Correspondence with systemic specialists such as dermatology, infectious disease or rheumatology are critical.6-10,13,15,16 In these cases, the classic topical regimen will require oral or intravenous supplementation which attacks the underlying disease process; including systemic corticosteroids and immunomodulating agents.6-10
In summery, MK has the potential to produce “sterile corneal infiltrates” and “sterile ulcerization”. MK itself is not an infectious process. It is an inflammatory response to a local toxic (chemical or microbial), mechanical, hypoxic or systemic inflammatory stimulus. The principle differential diagnoses for MK include microbial keratitis, Mooren’s ulcer, Terrien’s marginal degeneration, and peripheral keratolysis (peripheral ulcerative keratitis-sometimes referred to as corneal melting). Microbial keratitis (infectious corneal ulcer) is characteristically located centrally or paracentrally, is composed of a singular large lesion, is secondary to a unilateral process, produces an aggravated inflammatory response (iritis with cell and flare seen in the anterior chamber), is very painful and produces symptoms which include tearing, photophobia and decreased vision. Mooren’s ulcer is a painful, rapidly progressive keratitis that results in generalized peripheral corneal thinning, sometimes leading to perforation. Terrien’s degeneration is a bilateral, painless, progressive degeneration of the peripheral cornea occurring in the setting of an otherwise white and quiet eye. Corneal scrapings and cultures in MK are generally non-productive, even when the condition is associated with Staphylococcal blepharitis. Cultures should only be considered only if the condition does not improve within the first 48 to 72 hours of intervention. To avoid potential complications from occult fungal or herpetic infection, topical steroids are often prescribed under the protective umbrella of a concurrent topical antibiotic.
This patient was cyclopleged in the office with 1 % atropine OD so no cycloplegic drops would not have to be prescribed. They reduced the patient’s pain to minimal in 25 minutes. The patient was placed on a topical fourth generation fluoroquinolone QID, topical lubricants QID, bacitracin ointment HS to minimize the potential for recurrent erosion and instructed to use oral over-the- counter analgesics as tolerated and necessary for any residual pain. The patient was scheduled for a follow up evaluation in three days. Given the improvement and stability, topical 1 % prednisolone acetate was prescribed (added) QID, with a follow up evaluation scheduled for three days. The patient returned 90% improved. The regimen was continued for the balance of 10 days and rechecked. At the 10 day re-evaluation, now, at 100 % resolution, the topical antibiotic was discontinued and the topical steroid for the right eye tapered to BID for a week with orders to return for a final follow up. There were no complications at the final follow up, all medications were discontinued and the patient was discharged from care. A primary care exam was scheduled.
|
1. Zheng Y, Kaye AE, Boker A, et al. Marginal corneal vascular arcades. Invest Ophthalmol Vis Sci. 2013;54(12):7470-7. |

