Annual Innovations in Eye Care IssueFollow the links below to read other articles from our annual Innovations in Eye Care issue: Breaking the Burden: A New Way to Deliver Anti-VEGF |
Historically, visually significant endothelial dysfunction, most commonly caused by Fuchs’ endothelial dystrophy (FED), was treated by full-thickness corneal transplantation—penetrating keratoplasty (PK). Unfortunately, this procedure leaves the patient with a long visual recovery, a high chance of immunologic rejection, postoperative astigmatism, as well as the increased risk for wound dehiscence, even with minor ocular trauma years after the transplant.1
More recently, PK has been sidelined in favor of Descemet’s stripping endothelial keratoplasty (DSEK) and Descemet’s membrane endothelial keratoplasty (DMEK), partial-thickness transplant procedures that involve grafting of donor endothelium, with (DSEK) or without (DMEK) a small amount of donor stroma. Although visual recovery with these procedures is quicker than with traditional full-thickness PK, rejection and surgical complications still remain.2
With advances in surgical techniques over the past few decades, surgical outcomes for endothelial dysfunction patients have improved dramatically, but several significant problems remain in the developing world, namely a shortage of donor corneas and trained corneal surgeons. In the entire developing world, for example, fewer than 30,000 corneal transplants are performed yearly, while in the United States alone, almost 50,000 transplants were performed in 2016.3,4 This has driven research toward finding alternatives to the classic surgical approach of tissue transplantation.
 |
| One month postoperative appearance following 4mm Descemet’s stripping without endothelial keratoplasty in a 64-year-old man with Fuchs’ dystrophy. The arrow highlights the edge of the descemetorhexis. Click photo to enlarge. |
Several novel methods have been proposed to reduce the remaining complications of tissue transplantation for endothelial dysfunction, including the use of cultured human endothelial cells (which is potentially applicable for all forms of endothelial dysfunction) and Descemet’s stripping without endothelial keratoplasty, which eliminates the need for any donor tissue or cells, but is only applicable in the treatment of FED.2,5 A potentially important innovation is the use of rho-kinase (ROCK) inhibitors, either topically or intracamerally, as an adjunct to Descemet’s stripping or injection of cultured cells.5-8
This article briefly reviews our current understanding of the causes of endothelial dysfunction, and then delves into the status of these new and promising treatments.
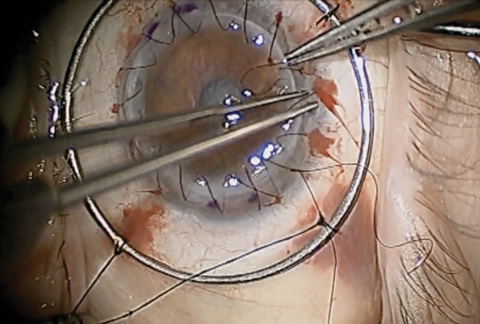 |
| Traditional PK, as seen here, comes with the risk of graft rejection, post-op astigmatism, suture management, intraocular complications and traumatic corneal rupture. Newer partial-thickness transplant procedures have helped minimize these potential complications. Click photo to enlarge. Photo: Derek N. Cunningham, OD, and Walter O. Whitley, OD, MBA |
Disease Basics
A firm grasp of the biology of the corneal endothelium is key to understanding the mechanisms that go awry in endothelial dysfunction. The corneal endothelium consists of a monolayer of hexagonal cells derived from neural crest cells. The endothelium maintains corneal transparency through the regulation of the hydration state of the cornea, which involves both a passive barrier function of the intact monolayer and active ATP-dependent ionic pumps.1 These cells are halted in the G1 phase of the cell cycle, and are therefore normally prevented from dividing after birth.
Any damage to the endothelial cells from trauma (e.g., following cataract surgery) or endothelial dystrophies, such as FED, causes the remaining healthy cells to undergo changes in shape (pleomorphism) and size (polymegethism), as they spread out to cover the damaged area.1 Corneal endothelial cell density in a healthy adult subject is between 2,000 and 2,500 cells/mm2. When the cell count drops to between 500 and 1,000 cells/mm2, the endothelium begins to decompensate, and corneal edema and reduced vision develop.6
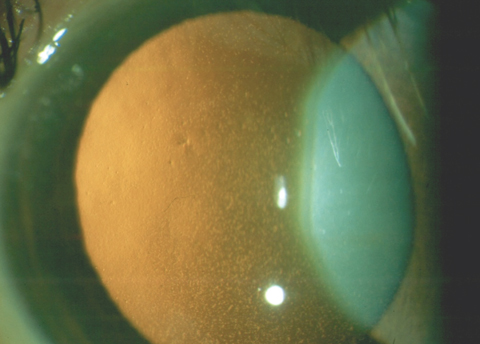 |
| This patient has confluent central guttae from FED. Even though the cornea retained normal thickness, the patient’s vision was reduced to 20/30 due to the visual degradation from the guttae. Click photo to enlarge. |
FED, the most common endothelial dysfunction, is usually diagnosed in patients older than 40. The disease is slowly progressive and generally requires intervention when patients reach their seventh or eighth decade of life. FED appears to follow an autosomal dominant inheritance pattern; however, older patient age during onset and variable penetrance makes a true determination of inheritance difficult. Many patients who are asymptomatic likely remain undiagnosed. Numerous genetic mutations have been associated with FED, including COL82A, SLC4A11, ZEB1, LOXHD1 and AGBL1.1 However, the most common genetic cause is expansion of a trinucleotide repeat in an intronic region of the TCF4 gene on chromosome 18, which accounts for up to 75% of cases in Caucasian patients.9
Corneal guttae—excrescences on or within Descemet’s membrane—are the initial finding of FED and can be found in up to 4% of patients in the United States, although a much smaller percentage of patients with guttae go on to develop FED.1 Guttae themselves scatter incoming light, causing decreased visual acuity, even without severe corneal edema.10
If left untreated, progressive FED leads to corneal edema, reduced visual acuity and, eventually, painful blindness. With the success of modern transplantation techniques, few patients in the developed world get to this advanced stage.
Many proposed mechanisms for FED exist, including mitochondrial dysfunction, oxidative stress and a variety of genetic mutations.1 Mitochondria play a vital role in the production of ATP, which is necessary to run the ionic pumps in the corneal endothelium that maintain proper corneal dehydration. Samples taken from human endothelial cells removed during keratoplasty have shown an increased number of mitochondria, suggesting a compensatory mechanism to combat mitochondrial dysfunction.11 The body naturally produces reactive oxygen species during the electron transport chain and as a response to UV exposure. When normal combative mechanisms against these substances break down, oxidative stress can damage DNA, proteins and lipids. Especially vulnerable to oxidative stress is mitochondrial DNA (mtDNA), which research shows can be damaged in the endothelium of FED patients compared with normal controls.12 Studies also show a reduction in telomere length, a common sign of DNA damage, in FED corneas, which further supports this theory.11
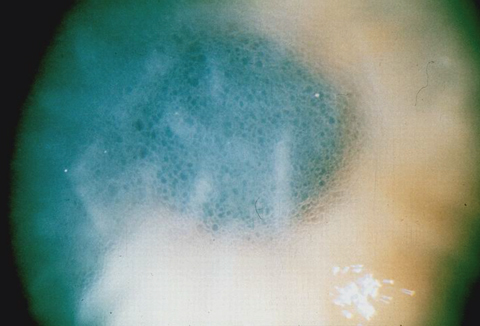 |
| This image shows advanced endothelial dysfunction with Descemet’s membrane folds and microcystic epithelial edema. Click photo to enlarge. |
Promising Novel Therapies
Given the significant visual effects of endothelial dysfunction—especially in FED where the guttae themselves can cause light scatter and visual degradation—and the potential complications associated with corneal transplantation, researchers continue to search for novel treatment options. A few are promising and have been used in a limited number of patients. Several of these new techniques may be enhanced by the adjuvant use of ROCK inhibitors.
Rho kinases are serine/threonine kinases that play an important role in diverse cellular pathways, including cell migration, proliferation and apoptosis.13 Two human rho-kinase homologs exist, ROCK1 and ROCK2, whose genes reside on different chromosomes, although they have similar functional domains. The distribution of the two ROCK homologs varies in different tissues, with ROCK2 predominating in the eye.
Researchers have looked at inhibition of the action of rho kinases by ROCK inhibitors as a treatment for a wide variety of disparate diseases, including asthma, cancer, osteoporosis and neuronal degeneration.14 In ocular disease, ROCK inhibitors are most commonly studied for glaucoma. Glanatec (ripasudil hydrochloride hydrate 0.4%, D. Western Therapeutics Institute), while not available in the United States, has been approved in Japan since 2014 for glaucoma and ocular hypertension.15 This agent inhibits both forms of rho kinase and is believed to act on the trabecular meshwork to increase outflow facility. In December 2017, the first topical ROCK inhibitor Rhopressa (netarsudil, Aerie Pharmaceuticals) was FDA approved as a treatment for glaucoma in the United States and should be available soon.
Research also shows ROCK inhibitors may play a role in the treatment of corneal endothelial dysfunction. Several animal studies demonstrate it can promote corneal endothelial healing in rabbit and monkey models.5,6 After Japanese researchers demonstrated that the ROCK inhibitor Y-27632 could promote primate corneal endothelial cell proliferation in vitro and healing in vivo in animal models, they sought to test the therapy on humans.
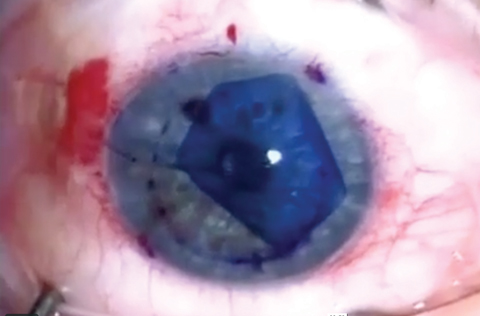
 |
| This patient was diagnosed with intermediate-stage FED with diffuse guttae and inferior stromal thickening, as demonstrated by the slit beam. Click photo to enlarge. |
Their 2013 case report described corneal clearance in FED after corneal endothelial denudation with cryotherapy followed by topical administration of Y-27632.8 In this case, a 52-year-old Japanese man, diagnosed with late-onset FED, was referred as a candidate for keratoplasty. Although his right eye was clear, his left cornea showed severe edema, his vision was 20/63 OS and his central corneal thickness was 703µm OS. His midperipheral endothelial cell count was maintained at a cell count of 757cells/mm2.
Transcorneal cryotherapy was used to destroy the central corneal endothelium and release the contact inhibition of the endothelial cells, thus allowing healthier cells in the periphery and mid-periphery to migrate to cover the denuded area. Y-27632 was compounded into an eye drop and used by the patient for one week following the endothelial destruction. By two weeks, vision had improved to 20/20 and the cornea had deturgesced. Corneal clarity was maintained throughout two years of follow-up. The researchers concluded that ROCK inhibitor eye drops may be a useful adjunct for less-invasive surgery (endothelial destruction without graft placement) for FED; however, they acknowledge they did not do the control of simply destroying the endothelium without the adjunct ROCK inhibitor and thus could not definitively isolate the effect of the ROCK inhibitor.
This approach (endothelial destruction without placement of a corneal graft) relies on the presence of functioning endothelial cells to cover the denuded area and would not be expected to succeed in situations characterized by global depletion of endothelial cells such as pseudophakic bullous keratopathy.
Several other less-invasive therapies are under development for the treatment of endothelial dysfunction, with ROCK inhibitors used as an adjunct with several of them.
 |
| Although advanced techniques such as DMEK have improved surgical outcomes for endothelial dysfunction patients, several novel methods—many used in conjunction with ROCK inhibitors—show promise for even better long-term visual outcomes. Click photo to enlarge. Photo: Jim Guzek, MD |
Cultured corneal endothelial cells (CECs). The same group of researchers in Japan has used CECs for transplantation following endothelial destruction in animal models.5 Engraftment of the transplanted CECs was facilitated by concurrent application of Y-27632. This team used monkeys whose corneal endothelium had been physically removed. Cultured monkey CECs (MCECs), in a suspension supplemented with Y-27632, were then injected into the anterior chamber, with the animal remaining in a face down position for three hours to allow the CECs to come in contact with the Descemet’s membrane. Results showed that the corneal endothelium that regenerated from the combined injection of CECs and ROCK inhibitor resembled normal corneal endothelial tissue, with a monolayer of hexagonal cells and Na+/K+ ATPases. There was no indication of adverse reactions from the injection, including intraocular pressure (IOP) increases, accumulations of injected cells or rejection.5
Following the successful regeneration of corneal endothelial tissue using MCECs, researchers then injected cultured human CECs (HCEC) into the monkey following endothelial removal, both with and without the ROCK inhibitor.5 While the HCEC injection without the ROCK inhibitor was unable to regenerate corneal endothelial tissue, a corneal endothelium was regenerated within one week after injection with HCECs along with the ROCK inhibitor.
Eyes injected with the HCECs and ROCK inhibitor showed corneal endothelium that resembled normal human endothelium, reaching a cell density of 2,890 cells/mm2. Again, no increase in IOP was observed, although secondary glaucoma is a potential side effect.
As of October 2016, this team had treated 31 patients with cultured cell injection therapy and is currently preparing their human data for publication.16 This approach opens up the possibility of dozens of CEC treatments from one donor cornea, helping to alleviate the corneal donor shortage. However, it also presents logistical challenges for regulatory approval in the United States, and peer-reviewed literature currently has little to offer on this approach in patients with endothelial dysfunction. Of course, this approach transplants donor cells and is a potential treatment for any form of endothelial dysfunction, since it does not require corneal rejuvenation by remaining host endothelium.
Rescued corneal endothelium without the use of foreign cells or tissue. The success of Descemet’s stripping without endothelial keratoplasty suggests this may be a possible treatment for FED in cases where host endothelial cells are preserved in the corneal periphery.2,7,17 In this procedure, the central area of the Descemet’s membrane with confluent guttae is simply removed, without placement of donor tissue. Peripheral endothelium migrates to cover the area of endothelial removal, providing corneal deturgescence without the need for foreign cells.1,2 This approach is only applicable in conditions where there are enough host endothelial cells are preserved to rejuvenate the cornea, most commonly in FED. It will not be effective in conditions characterized by complete endothelial depletion such as pseudophakic bullous keratopathy or advanced FED.
The first large series to show success with this approach studied Descemet’s stripping after cataract removal and intraocular lens insertion in patients with moderate-stage FED.2 In this study, corneal clearance occurred in 10 of 13 cases after a 4mm central Descemet’s stripping, at an average of three months (range one to six months). The three eyes that failed to clear underwent standard endothelial transplantation with good outcomes.18
Others have also confirmed the viability of Descemet’s stripping without endothelial replacement as a treatment for FED, although it has shown variable success in a few small series by other authors.2 Recently, one study confirmed corneal clearance in 14 of 17 FED patients after Descemet’s stripping without endothelial replacement, but demonstrated a much greater success rate (10 of 10 cases) if Descemet’s membrane and endothelium were removed by a smooth tear method known as descemetorhexis, rather than scoring and subsequent Descemet’s stripping. This suggests physical characteristics of the area of endothelial removal (i.e., smooth rather than jagged edge) may play a role in the success of this technique.17
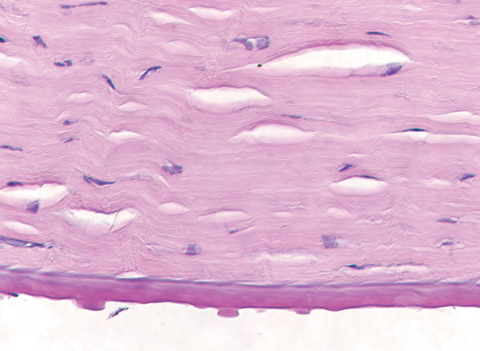 |
| This specimen, removed following corneal transplantation, shows the histopathology of corneal guttae. Note the Descemet’s membrane thickening, also a hallmark of FED. Click photo to enlarge. |
A recent study of Descemet’s stripping without grafting in 12 FED eyes suggests the use of the ROCK inhibitor ripasudil as a rescue agent for patients whose corneas did not clear after endothelial removal alone.7 In this study, nine of 12 eyes cleared spontaneously. One eye that did not clear was treated with compounded topical Y-27632 without success and eventually underwent endothelial keratoplasty with a good outcome. Two eyes had persistent edema with stalled corneal deturgescence two and three months after the Descemet’s stripping. The authors applied ripasudil to these two cases and noted clearing of the corneal edema after the drop was used six times daily for two weeks.
Since this study was published in 2017, other groups have started to examine the effect of topical ROCK inhibitors as an adjunct to endothelial removal or destruction as a treatment for FED. None of these studies have reached the peer-reviewed literature yet, but preliminary data suggests topical ripasudil speeds endothelial migration (corneal clearance in six weeks rather than three months), reduces the number of cases that fail to clear and may increase the final central endothelial cell count.
Many questions remain, however, including the optimal dose and duration of ROCK inhibitors, as well as which agent is best. The recent approval of netarsudil in the United States should help the eye care community discover answers to these questions.
While modern corneal transplantation techniques are highly successful for management of endothelial failure, several novel therapies have shown promise in recent years, especially for Fuchs’ dystrophy, the most common cause of endothelial dysfunction. More work is needed to determine the optimal patient population for Descemet’s stripping without endothelial keratoplasty, as well as the role of topical rho-kinase inhibitors in this procedure. Cultured endothelial cells offer the possibility of treating many patients from a single donor, although significant logistical obstacles stand in the way of widespread acceptance of this approach.
These techniques have the potential to reduce the risks associated with conventional surgical treatments, may offer options for earlier-stage endothelial dysfunction patients, especially those with Fuchs’ dystrophy, and are likely to play an increasing role in the management of corneal endothelial diseases in the future.
Ms. Rohan is a second-year optometry student at the Illinois College of Optometry.
Dr. Colby is the Louis Block Professor and chairman of the Department of Ophthalmology and Visual Science at the University of Chicago. An internationally renown corneal surgeon, Dr. Colby is an expert in Fuchs’ dystrophy and has pioneered the use of Descemet’s stripping for FED management.
1. Sarnicola C, Farooq A, Colby K. Fuchs endothelial corneal dystrophy: Update on pathogenesis and future directions. Eye & Contact Lens. 2018, in press. |

