Retinal detachment (RD)—a serious condition that requires urgent attention—is commonly encountered in vitreoretinal subspecialty practices. Rhegmatogenous retinal detachment (RRD) is the separation of the neurosensory retina from the underlying retinal pigment epithelium (RPE) by fluid traversing from the vitreous cavity into the subretinal space via a retinal defect (Figure 1). The intervening time between the development of a retinal defect and retinal detachment is highly variable and often unpredictable. Even giant tears may sit idly without progressing to a detachment for an extended period of time.
The preliminary or “triage” diagnosis is made based on the presenting history—such as sudden onset flashes, floaters, vision loss or a combination—as well as clinical and ancillary examination. At times, these presenting examination findings can be misleading, resulting in misdiagnosis and mismanagement of the patient.
Here are the principles of RRD, including pathophysiology, symptomatology, clinical findings and surgical management.
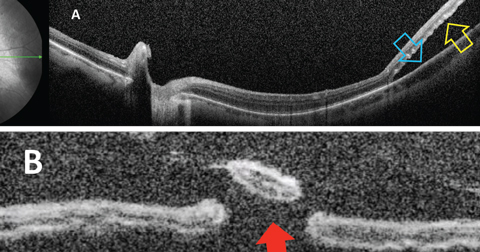 |
| Fig. 1. Optical coherence tomography showing (A) separation of neurosensory retina (yellow arrow) from the RPE (blue arrow); (B) percolated retinal break (red arrow). Click image to enlarge. |
Epidemiology and Etiology
All non-exudative and non-tractional RDs originate from a retinal defect. Retinal defects are commonly described as tears or holes, and the most common way to acquire one is through the development of a posterior vitreous detachment (PVD).1-4 Vitreous degeneration as a process of aging is well understood and is best described as the progressive detachment of the vitreous cortex from the retinal surface, which rarely creates retinal tears. Progression time from tear to detachment is unpredictable, and varies from hours to days or even months. Some tears may exist chronically for years, while others create detachments in a matter of hours. Most tears, if caught early, can be treated with a laser in a clinical setting even with the presence of a small amount of subretinal fluid. Once a retinal detachment develops, surgical intervention becomes necessary.
Risk factors other than age include: family history, particularly in certain genetic conditions, high axial myopia, history of RRD in the fellow eye, trauma, certain conditions such as uveitis and retinopathy of prematurity (ROP). Previous intraocular surgery, especially for cataract, is a risk factor.5,6
The incidence of RD in the general population is between 0.01% and 0.018%.5-15 This increases to 1% following cataract surgery and up to a four-fold increase with Nd:YAG capsulotomy.16-21 Peripheral retinal disease and degeneration can be a predisposing risk factor for RRD, especially lattice degeneration (Figure 2).3,22 Approximately 30% of phakic patients with RRD have pre-existing lattice degeneration, whereas all patients with lattice degeneration have less than 1% chance of developing RRD.23,24
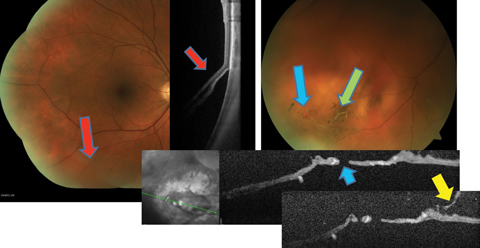 |
| Fig. 2. This patient with preexisting lattice (green arrow), developed a retinal break (blue arrows) during PVD (yellow arrow), resulting in an inferior retinal detachment (red arrows). Click image to enlarge. |
Symptomatology
Asymptomatic RDs are usually detected during primary eye care visits. These usually present as inferior or temporal chronic detachments originating from atrophic holes (Figure 3). A non-central RD can be asymptomatic, as patients may not be as sensitive to slowly progressive peripheral vision loss. This is particularly common when RRDs occur in inferior and temporal locations because the superior and nasal fields of view are less sensitive.
Most patients presenting with an RD report recent positive visual phenomenon despite the detachment’s onset possibly being weeks to months prior to the presentation of symptoms. There are many causes for this: patients do not notice flashes if they are asleep, recognition of unilateral vision loss is often delayed and RDs are usually first noticed only when they begin to affect central vision.
Symptoms may also be nonspecific. Floaters, a common problem across all age groups, are usually benign. Flashes may be associated with successful vitreous separation without retinal tears or detachment or may have non-ocular etiologies such as transient ischemic attack and acephalgic migraine. Negative dyschromatopsia may simulate flashes and visual field loss, and various other intraocular processes may decrease central and peripheral vision. Still, complaints of flashes, floaters or negative dyschromatopsia warrant a thorough evaluation, as any of these symptoms may be associated with RRD. With uncertain or unusual findings, clinicians should refer to a specialist.
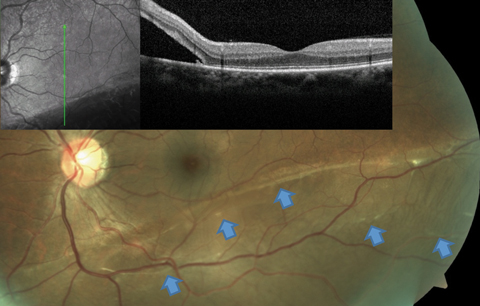 |
| Fig. 3. This RRD patient’s OCT image, layered over a fundus photograph, shows partial involvement of the macula, even though the patient is totally asymptomatic from this slowly progressive inferior RD. Blue arrows point to a number of subretinal bands and partial demarcation lines, a testament to the chronicity of the condition. Click image to enlarge. |
Findings
The clinical evaluation should be as thorough as possible, especially the dilated fundus exam. A macula-involving RD should be easy to spot, but a far peripheral detachment, especially in the pediatric population, can be quite challenging to identify. Although the goal is to identify retinal abnormalities before they progress to detachment, if you observe any hint of subretinal fluid (SRF) or a retinal break with or without SRF, you’ve seen all that is needed to warrant an immediate referral. While the retinal specialist will document every break, the referring doctor can help document as many as possible before the patient sees the specialist.
If the clinical exam does not reveal an RD, scleral depression and a three-mirror contact lens examination can help to rule out small peripheral tears. Again, if you have any doubt, referral is always the best option. The presence of acute PVD, pigment cells in the anterior vitreous or vitreous hemorrhage should increase the level of suspicion and calls for further examination, either by ultrasonography or vitrectomy.1,2,4,25-27 In particular, retinal breaks are highly probable with vitreous hemorrhage secondary to PVD, and need to be discovered or ruled out by careful examination.
Certain conditions may present similarly to RRD, including: serous retinal detachment secondary to inflammatory or neoplastic disease, tractional retinal detachment, vitreous opacities such as vitreous hemorrhage as well as retinoschisis (Figure 4).
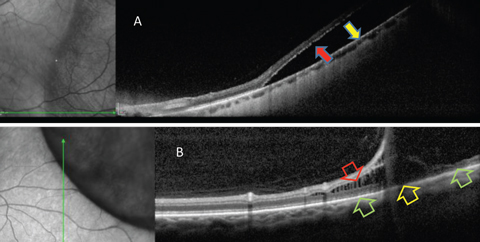 |
| Fig. 4. OCT is helpful in differentiating RRD from retinoschisis. (A) Neurosensory retina is separated from RPE in RRD. This patient requires surgical intervention. (B) In retinoschisis, separation and spilling of neurosensory is seen. Normal apposition of the outer retina to RPE is seen (green arrows) on either side of the outer retinal break (yellow arrow). This patient may be closely monitored. Click image to enlarge. |
Auxiliary Testing
Though the most effective technique to diagnose RRD is a thorough dilated fundus exam, certain ancillary tests can also help clinicians better document the severity of the retinal abnormality. Widefield fundus photography, often with conventional white flash, can help, as can scanning laser ophthalmoscopy devices. Optical coherence tomography (OCT), particularly in widefield mode, is helpful in the differential diagnosis of RRD and can help clinicians assess the macular status at the time of diagnosis.28 Surgical intervention is more time-sensitive in macula-on as opposed to macula-off detachments. This role is reversed in tractional RD, where macular involvement requires quicker surgical intervention. Using B-scan ultrasound is usually not necessary to diagnose RRD when the clinical view is clear; however, it is essential in the presence of media opacification, as well as in the
differential diagnosis of masquerading conditions.29
Fluorescein angiography (FA) and visual field (VF) testing are also not essential, though they may aid in the differential diagnosis. VFs may be necessary in cases of injury, workers’ compensation, litigation or assessment of functional vision loss.
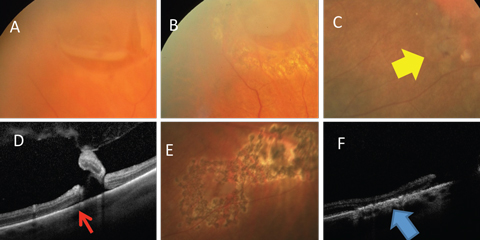 |
| Fig. 5. (A) Horseshoe retinal tear is well bordered by laser (B). (C) Retinal breaks (yellow arrow) with (D) associated subretinal fluid (red arrow) well bordered by (E) laser. (F) Alteration of the RPE resulting in a watertight tissue (blue arrow). Click image to enlarge. |
Management Options
Timely management of RRD and its associated findings is critical to decrease the chance of long-term vision loss.
Round holes, horseshoe tears and giant breaks can all be treated with laser or cryotherapy in an office equipped and staffed for it (Figure 5).30,31 Small amounts of associated fluid may also be surrounded with laser or cryotherapy to prevent progression. The presence of substantial subretinal fluid warrants either a pneumatic retinopexy or operative RD repair. Localized, slow-growing chronic RDs, which are usually described as subclinical based on their usually asymptomatic nature may be walled off with laser as well (Figure 6).32 Laser treatment is performed with either a slit lamp laser or laser indirect ophthalmoscopy (LIO). Both are highly effective; however, LIO is usually required for far peripheral treatment.
Laser treatments are only effective in regions where neurosensory retina and the RPE are either in normal apposition or when they can be brought together through scleral depression; therefore, the laser is applied at the border of the attached and detached retina. Scleral depression can help to drive the fluid away by increasing the surface area on which the laser can be applied.
Although the consensus is to monitor asymptomatic retinal tears, there is still a 5% chance of RD with need for more complex management and potential of vision loss.33 This statistic, we believe, makes a strong case for treating all retinal defects with laser treatments to guard against RD. Horseshoe tears should be treated immediately, while round holes are less urgent. Prophylactic retinopexy is normally an extremely low risk procedure when performed by a skilled operator, and in absence of retrobulbar block.33
Pneumatic retinopexy is best used for RDs where the retinal break is localized to the superior 180 degrees of the retina and, if more than one break exists, they are localized in such a way that an expansible bubble can adequately cover them with proper patient positioning.30 However, this technique is not effective in the presence of proliferative vitreoretinopathy (PVR). Pneumatics may also be used in some cases as a temporary tamponade when an operating room procedure is delayed for logistical reasons. The procedure involves carefully positioning the patient, then injecting a bubble of pure perfluoropropane (C3F8) or sulfur hexafluoride (SF6) though the pars plana. The anterior chamber is either tapped prior to or immediately after the gas is injected to normalize the intraocular pressure (IOP). Retinopexy can be performed as either cryotherapy, which has to be applied to the breaks prior to gas injection, or with a laser, which is performed after several hours or days once the retina is re-attached.
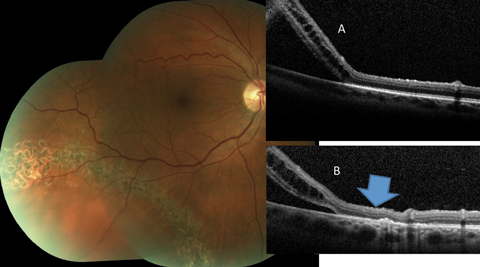 |
| Fig. 6. This asymptomatic patient with subclinical RRD not involving the macula is effectively treated by laser bordering. The alteration of the outer retina-RPE junction can be noted when the pretreatment OCT (A) is compared to the posttreatment scan (B) (blue arrow). Click image to enlarge. |
Procedural Pearls
The selection between C3F8 or SF6 depends on the individual surgeon’s preference and comfort with each gas. C3F8, which is a long-acting fluorinated gas that expands to a greater volume than SF6, is used for all pneumatic retinopexies we perform. However, in our practice we use an iso-expansile SF6/air mixture for all rhegmatogenous RDs needing vitrectomy. Complications related to gas use in vitreoretinal surgery include: increased intraocular pressure in the short term (due to incorrect gas mix or pupil block in aphakia, for example) and cataract formation in the medium term (due to incorrect patient positioning). The self-elimination could also be a weakness, mainly due to the insufficient time for chorioretinal adhesion formation, resulting in failure of the primary surgery.
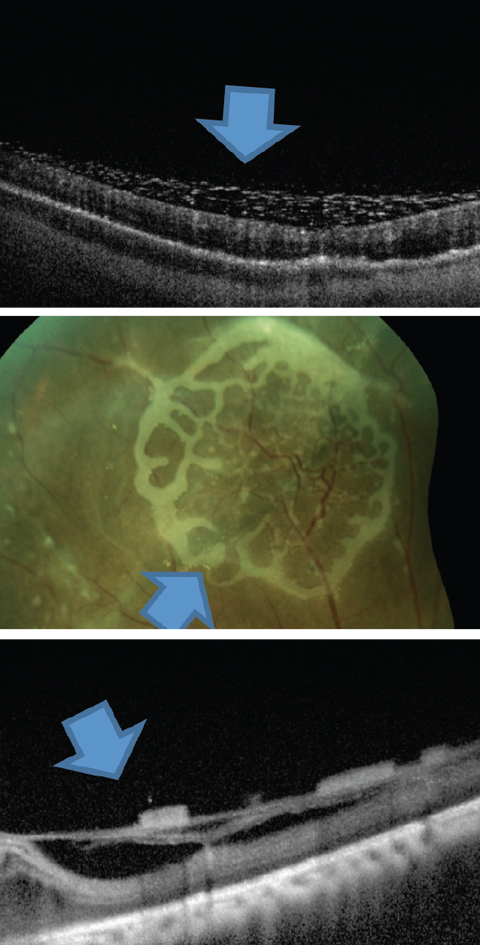 |
| Fig. 7. This is one presentation of emulsified silicone seen on the retinal surface. Click image to enlarge. |
Small-incision pars plana vitrectomy (PPV) using 25- or 27-gauge instruments is a safe and effective surgical procedure.34,35 In RRD repair, several agents in addition to C3F8 and SF6, such as silicone oil or the perfluorocarbon liquid perfluoron (PFO) are used for retinal tamponade.36 The attraction between the individual molecules in these substances creates surface tension, which prevents aqueous egress back through retinal defects and prevents retinal re-detachment immediately after surgery to give the laser the time needed to form a strong chorioretinal scar. Factors that influence the choice of agent include: location and extent of the RD and the associated retinal breaks, presence of other clinical findings such as PVR and the patient’s ability to position.
Silicone oil is a good option when the likelihood of retinal re-detachment is high in the immediate postoperative period due to an inability to adequately treat retinal breaks (albinism, blood-obscuring known breaks, loss of visualization in surgery), an inability to correctly position and PVR, for example.
Unlike C3F8 and SF6, which are slowly absorbed out of the vitreous cavity and replaced with aqueous, silicone oil can remain in the vitreous cavity for a long time. However, the long-term duration of silicone can result in some complications, including shearing off multiple small bubbles through the detergent action of intraocular proteins and patient motion, which is termed silicone emulsification (Figure 7). These bubbles may then migrate to the anterior chamber and cause blockage of the trabecular meshwork, resulting in increased IOP and subsequent glaucoma or corneal decompensation. Silicone oil removal or replacement requires another trip to the retinal surgeon.
The final vitreous substitute is PFO. This is a liquid with high specific gravity that will sink to the inferior vitreous cavity. PFO can be used as an intraoperative tool to unfold the retina in giant RDs, as well as to drain subretinal fluid out the original tears without creation of a retinotomy. PFO can also be used in the medium term by leaving the PFO in for one to two weeks after performing laser retinopexy around all breaks for the laser to heal. Another trip to the surgeon is required for removal. While an uncommon technique, it works in the right situation. PFO causes a modest foreign body inflammatory reaction in some patients if used on a medium-term basis or if a small amount is retained after surgery. PFO will also migrate to the subretinal space if used inappropriately for tractional RDs or PVR.30 If droplets are retained in the anterior chamber, they may be removed at the slit lamp in the office relatively easily.
Significant RDs are treated either with vitrectomy alone or scleral buckle with or without vitrectomy.30 The use of scleral buckle has decreased in favor of vitrectomy.37,38 The discomfort, poor refractive outcomes and tissue erosion caused by scleral buckles can be avoided with vitrectomy alone without compromising outcome rates.39,40 No studies show scleral buckle is superior to vitrectomy-based surgery alone. Scleral buckling induces unpredictable myopic shift, pain, increased phorias and tropias, buckle extrusion, ptosis and ocular surface disorder. Nevertheless, good outcomes are still achieved with scleral buckle, and most retina surgeons still advocate their use in subclinical RDs without proliferative vitreoretinopathy in children and young adults.
Regardless of tamponade agent or use of a buckle or not, the most common cause of RRD repair failure is PVR.30,41 Other causes include inadequate retinopexy, poor patient positioning and unrelieved vitreous traction resulting in additional tears forming after leaving the operating room.
Complications following vitrectomy include increased rate of progression of cataract, endophthalmitis, suprachoroidal hemorrhage and re-detachment.42
RRDs, without proliferative vitreoretinopathy, can have single surgery success rates as high as 75% with pneumatic retinopexy, and up to 90% with vitrectomy, scleral buckling or a combination of the two.35 Proliferative vitreoretinopathy at presentation implies chronicity and a higher likelihood of requiring multiple surgeries. The crucial factors in obtaining good outcomes include timely diagnosis, selection of the right intervention and careful follow up. Optometrists play a significant role as the primary eye care providers and the entry point of most patients with signs and symptoms suggestive of retinal detachment.
Dr. Rafieetary is a consultative optometrist at the Charles Retina Institute in Germantown, Tenn.
Dr. Huddelston is a vitreoretinal surgeon at the Charles Retina Institute in Germantown, Tenn.
Dr. Attar is an assistant clinical professor at the University of Pikeville, Kentucky College of Optometry.
The authors would like to thank Steve Charles, MD, for his contribution to this article.
| 1. Boldrey EE. Risk of retinal tears in patients with vitreous floaters. Am J Ophthalmol. 1983;96:783-7. 2. Brod RD, Lightman DA, Packer AJ, Saras HP. Correlation between vitreous pigment granules and retinal breaks in eyes with acute posterior vitreous detachment. Ophthalmology. 1991;98:1366-9. 3. Byer NE. Long-term natural history of lattice degeneration of the retina. Ophthalmology. 1989;96:1396-401. 4. Dayan MR, Jayamanne DG, Andrews RM, Griffiths PG. Flashes and floaters as predictors of vitreoretinal pathology: Is follow-up necessary for posterior vitreous detachment? Eye. 1996;10:456-8. 5. Brown DM, Graemiger RA, Hergersberg M, et al. Genetic linkage of Wagner disease and erosive vitreoretinopathy to chromosome 5q13-14. Arch Ophthalmol. 1995;113:671-5. 6. Snead MP, Payne SJ, Barton DE, et al. Stickler syndrome: Correlation between vitreoretinal phenotypes and linkage to COL 2A1. Eye. 1994;8(Pt 6):609-14. 7. Feltgen N, Walter P. Rhegmatogenous retinal detachment - an ophthalmologic emergency. Dtsch Arztebl Int. 2014;111:1-2. 8. Haimann MH, Burton TC, Brown CK. Epidemiology of retinal detachment. Arch Ophthalmol. 1982;100:289-92. 9. Ivanisevi M, Boji L, Eterovi D. Epidemiological study of nontraumatic phakic rhegmatogenous retinal detachment. Ophthalmic Res. 2000;32(5):237-9. 10. Van de Put MA, Hooymans JM, Los LI. The incidence of rhegmatogenous retinal detachment in The Netherlands. Ophthalmology. 2013;120:616-22. 11. Wilkes SR, Beard CM, Kurland LT, et al. The incidence of retinal detachment in Rochester, Minnesota, 1970-1978. Am J Ophthalmol. 1982;94:670-3. 12. Eye Disease Case-Control Study Group. Risk factors for idiopathic rhegmatogenous retinal detachment. Am J Epidemiol. 1993;137(7):749-57. 13. Byer NE. Rethinking prophylactic therapy of retinal detachment. In: Stirpe M, ed. Advances in Vitreoretinal Surgery. New York, NY: Ophthalmic Communications Society; 1992:399-411. 14. Cooling RJ. Traumatic retinal detachment—mechanisms and management. Trans Ophthalmol Soc UK. 1986;105:575-9. 15. Kaiser RS, Trese MT, Williams GA, Cox MS Jr. Adult retinopathy of prematurity: Outcomes of rhegmatogenous retinal detachments and retinal tears. Ophthalmology. 2001;108:1647-53. 16. Erie JC, Raecker MA, Baratz KH, et al. Risk of retinal detachment after cataract extraction, 1980-2004: A population-based study. Ophthalmology. 2006;113:2026-32. 17. Jahn CE, Richter J, Jahn AH, et al. Pseudophakic retinal detachment after uneventful phacoemulsification and subsequent neodymium: YAG capsulotomy for capsule opacification. J Cataract Refract Surg. 2003;29:925-9. 18. Olsen, T, Jeppeen P. The incidence of retinal detachment after cataract surgery. Ophthalmol J. 2012;6:79-82. 19. Russell M, Gaskin B, Russell D, Polkinghorne PJ. Pseudophakic retinal detachment after phacoemulsification cataract surgery: Ten-year retrospective review. J Cataract Refract Surg. 2006;32:442-5. 20. Javitt JC, Tielsch JM, Canner JK, et al. Cataract Patient Outcomes Research Team. National outcomes of cataract extraction: increased risk of retinal complications associated with Nd:YAG laser capsulotomy. Ophthalmology. 1992;99:1487-98. 21. Tielsch JM, Legro MW, Cassard SD, et al. Risk factors for retinal detachment after cataract surgery: A population-based case-control study. Ophthalmology. 1996;103:1537-45. 22. Benson WE, Morse PH. The prognosis of retinal detachment due to lattice degeneration. Ann Ophthalmol. 1978;10:1197-200. 23. Sasaki K, Ideta H, Yonemoto J, et al. Risk of retinal detachment in patients with lattice degeneration. Jpn J Ophthalmol. 1998;42(4):308-13. 24. Wilkinson CP. Interventions for asymptomatic retinal breaks and lattice degeneration for preventing retinal detachment. Cochrane Database of Systematic Reviews. 2012;3:CD003170 25. Byer NE. Natural history of posterior vitreous detachment with early management as the premier line of defense against retinal detachment. Ophthalmology. 1994;101:1503-14. 26. Coffee RE, Westfall AC, Davis GH, et al. Symptomatic posterior vitreous detachment and the incidence of delayed retinal breaks: Case series and meta-analysis. Am J Ophthalmol. 2007;144:409-13. 27. Tasman WS. Posterior vitreous detachment and peripheral retinal breaks. Trans Am Acad Ophthalmol Otolaryngol. 1968;72:217-24. 28. Uchino E, Uemura A, Ohba N. Initial stages of posterior vitreous detachment in healthy eyes of older persons evaluated by optical coherence tomography. Arch Ophthalmol. 2001;119:1475-9. 29. DiBernardo C, Blodi B, Byrne SF. Echographic evaluation of retinal tears in patients with spontaneous vitreous hemorrhage. Arch Ophthalmol. 1992;110:511-4. 30. Charles S, Calzada J, Wood B. Vitreous Micro Surgery, 5th edition. Baltimore: Lippincott Williams & Wilkins; 2011. 31. Davis MD. Natural history of retinal breaks without detachment. Arch Ophthalmol. 1974;92:183-94. 32. Kazahaya M. Prophylaxis of retinal detachment. Semin Ophthalmol. 1995;10(1):79-86. 33. Silva RA, Blumenkranz MS. Prophylaxis for retinal detachments. American Academy of Ophthalmology. Clinical Education. Published October 2013. Accessed August 23, 2017. www.aao.org/munnerlyn-laser-surgery-center/prophylaxis-retinal-detachments. 34. Sato T, Emi K, Bando H, Ikeda T. Faster recovery after 25-gauge microincision vitrectomy surgery than after 20-gauge vitrectomy in patients with proliferative diabetic retinopathy Clin Ophthalmol. 2012;6:1925-30. 35. Khanduja S, Kakkar A, Majumdar S, et al. Small gauge vitrectomy: Recent update. Oman J Ophthalmol. 2013 Jan-Apr;6(1): 3-11. 36. Simone D, Simona MC, Giulia A, et al. Vitreous substitutes: The present and the future. BioMed Res Intern. 2014. 37. Chong DY, Fuller GD. The declining use of scleral buckling with vitrectomy for primary retinal detachments. Arch Ophthalmol. 2010;128(9):1206-7. 38. Baseer U. Ahmad, MD, Gaurav Shah, MD, and Kevin Blinder Trends & Approaches to Repairing Detachment. Review of ophthalmology April 2013 39. Hammersley J, Covert D, Han D, et al. Outcomes and complications of scleral buckle removal. Invest Ophthalmol Vis Sci. 2009;50:2063. 40. Ambati J, Arroyo JG Postoperative complications of scleral buckling surgery. Int Ophthalmol Clin. 2000 Winter;40(1):175-85. 41. Leaver PK. Proliferative vitreoretinopathy. British J Ophthalmol. 1995;79(10):871–2. 42. Roe RH, McDonald RH. Complications of vitreoretinal surgery. Rev Ophthalmol. 2008;13(10). |

