Annual Retina ReportCheck out the other feature articles in this month's report:Sizing Up Geographic Atrophy Macula Exam Tips and Tricks Setting Patient Expectations for Anti-VEGF Therapy Tracing Clues Back to Retinitis Pigmentosa |
The American Optometric Association (AOA) published its most recent evidence-based guidelines on how to care for patients with diabetes in October 2019.1 These guidelines suggest referring patients with diabetes who present with severe or very severe nonproliferative diabetic retinopathy (NPDR), early proliferative diabetic retinopathy (PDR) with risk of progression or high-risk PDR.1
In an effort to prevent diabetic retinopathy (DR) from progressing to the point of referral, current and recent clinical trials have been exploring a new, earlier treatment strategy relying on anti-VEGF injections. This article provides an overview of ocular conditions associated with diabetes, discusses the success anti-VEGF treatment is achieving and offers a protocol for optometrists who work closely with these patients.
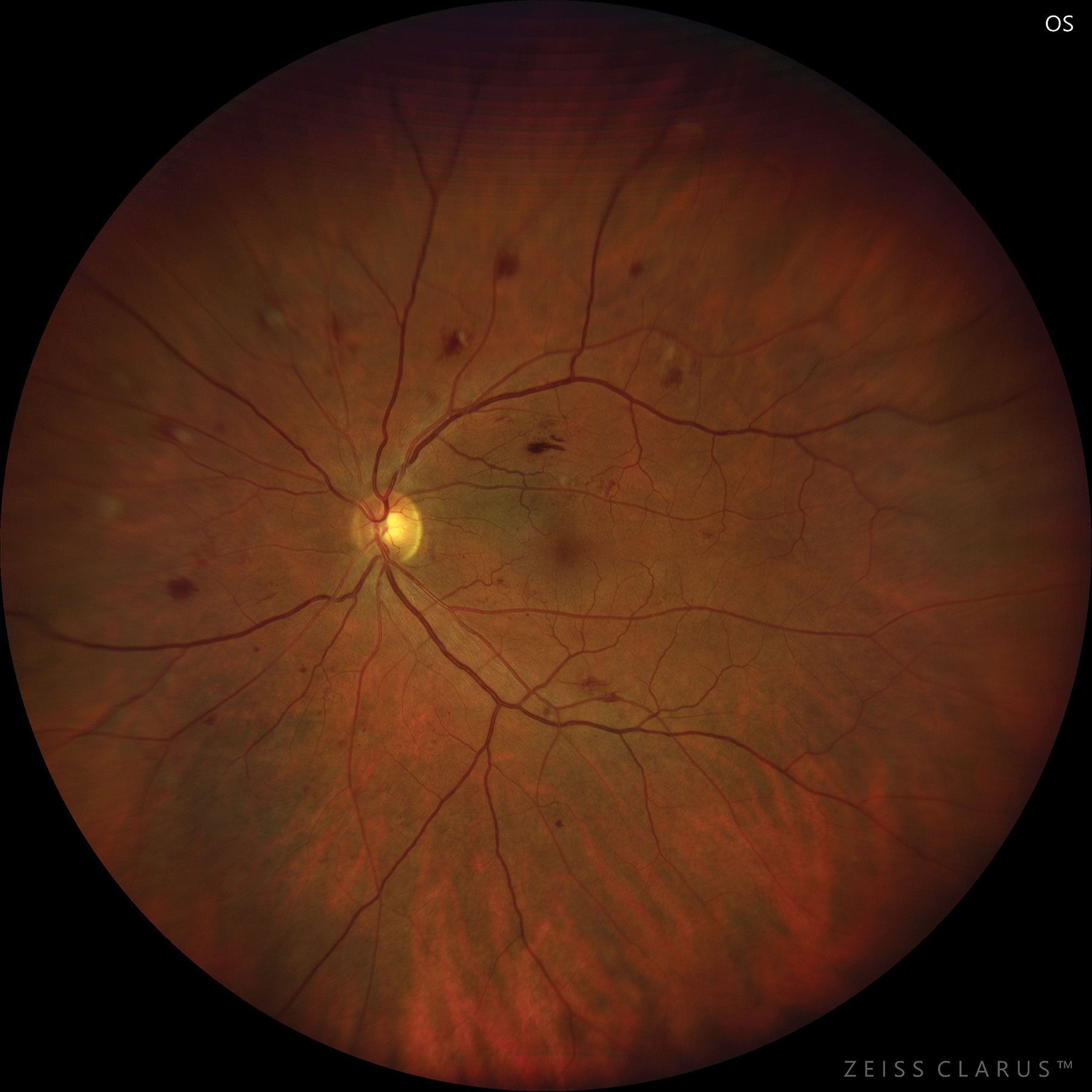 |
| Moderate NPDR care consists of several management techniques, as there is currently no treatment. Photo: Jay M. Haynie, OD. Click image to enlarge. |
The Basics
Globally, 35% of patients with diabetes have DR, and 10% have a vision-threatening form, such as PDR, severe NPDR or diabetic macular edema (DME).1 In the United States alone, 40% of adults older than 40 with diabetes have DR, and 8% have vision-threatening DR.1
The early physiological change indicative of DR is decreasing retinal blood flow, or macular ischemia. The imbalance of nitric oxide and endothelin-1 levels induces retinal vessel spasm, leading to blood flow reduction and tissue ischemia in the retina.2 Visible leaking retinal capillaries are a sign the inner and outer blood-retinal barriers are breaking down.
Macular ischemia is associated with a poor visual prognosis and an even poorer response to treatment.3 It is defined as widening of the foveal avascular zone, disruption of the perifoveal capillary net and capillary non-perfusion in the central macula (within one disc-diameter of the foveal center).4 Areas of capillary non-perfusion indicate the severity of retinopathy and the risk of neovascularization. RECOVERY, an ongoing Phase II trial, aims to assess the effect of anti-VEGF injections every one or three months on retinal capillary non-perfusion in PDR over a one-year period.5
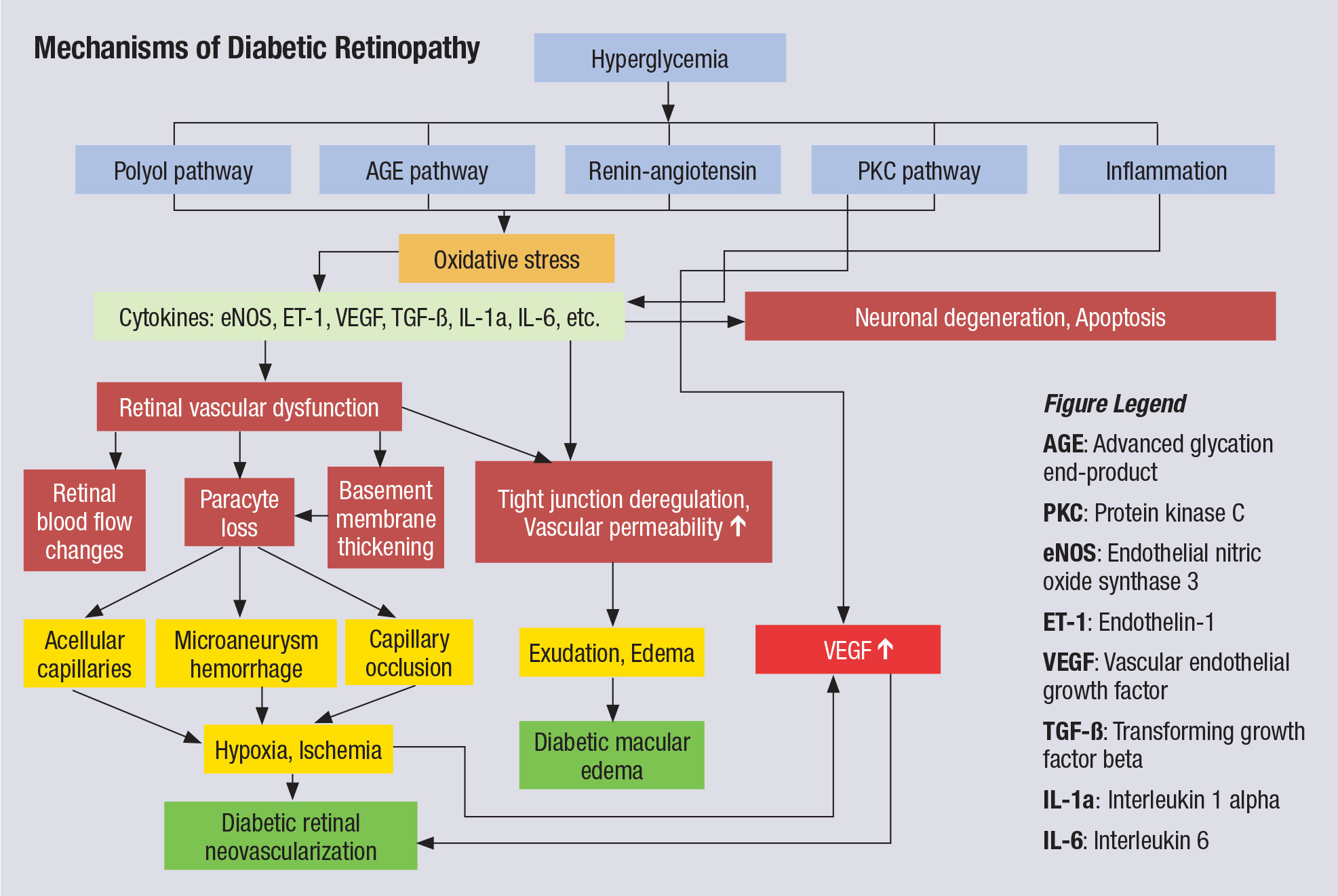 |
| This chart illustrates the complex pathophysiology of DR. Note that VEGF elevation happens fairly late in the cascade and directly before neovascularization, which partly explains why anti-VEGF therapy must be maintained indefinitely. Other interventions, especially those that might blunt activity further upstream from VEGF, could have more long-lasting impact. Click image to enlarge. |
DME is diagnosable if retinal thickening is present or hard exudates exist within one disc-diameter of the center of the macula. The Early Treatment Diabetic Retinopathy Study (ETDRS) defines clinically significant macular edema (CSME) as DME with at least one of three criteria: thickening of the retina at or within 500µm of the center of the macula, hard exudates at or within 500µm of the center of the macula associated with thickening of the adjacent retina or an area of retinal thickening one disc-diameter or larger, part of which is within one disc-diameter of the center of the macula.6
There are two kinds of edema: focal and diffuse. Focal edema is induced by a focal leakage from microaneurysms, while diffuse edema is caused by retinal capillary leakage (abnormal permeability).7
DME is classified as center involved (CI) DME if retinal thickening greater than 250µm is present in the central subfield zone of the macula or non-CI DME if retinal thickening exists inside the macula but outside the central subfield zone. This classification strategy is becoming the more accepted way of diagnosing DME in a clinical setting. ETDRS showed that CI DME is 10 times more likely than non-CI DME to cause vision loss.6
The pathological changes of DR and DME include both angiogenic and inflammatory processes. The molecular mechanisms of DR and DME consist of hyperglycemia-induced oxidative stress, the sorbitol pathway, advanced glycation end-products, the renin-angiotensin system, diacylglycerol-protein kinase C pathway activation, inflammatory markers and raised VEGF levels.8 Chronic hyperglycemia and hypertension lead to oxidative injury, microthrombi formation, cell adhesion, molecule activation, leukostasis and cytokine activation, causing further retinal damage.9
 |
| Anti-VEGF may help improve visual outcomes for cases of moderate to severe NPDR. Photo: Jay M. Haynie, OD. Click image to enlarge. |
Treatment Outlook
Ischemia can induce VEGF production. VEGF causes angiogenesis and induces retinal neovascularization in diabetes and choroidal neovascularization in age-related macular degeneration (AMD). It also increases the permeability of the blood-retinal barrier by down-regulating the tight junctions of the endothelium of the retinal vessels.
Intravitreal injections of anti-VEGF agents reduce neovascularization and vessel leakage.10 The efficacy of injection-only treatment (success rate of 30% to 50%) surpasses that of laser treatment in patients with DME.11,12
Four anti-VEGF agents are currently available. Avastin (bevacizumab, Genentech) is FDA-approved for cancer treatment and has been used off-label for several retinal diseases, including DME. Lucentis (ranibizumab, Genentech) is FDA-approved for treating wet AMD, retinal vein occlusion and DR with or without DME. Eylea (aflibercept, Regeneron) is FDA-approved for the treatment of wet AMD, central retinal vein occlusion, DME and DR without DME. Beovu (brolucizumab-dbll, Novartis) is approved to treat wet AMD.
While some retina specialists may consider surgical treatment with lasers if a pre-CSME case meets certain criteria, recently more of an emphasis is being placed on proactively treating these patients with anti-VEGF. Earlier treatment with these intravitreal injections could prevent vision-threatening complications from developing later on, reduce the need for potential future treatment and promote better visual acuity.
Anti-VEGF may also be an effective treatment option for vitreous hemorrhage (VH) secondary to DR, which can induce vision loss. Anti-VEGF and vitrectomy combined with panretinal photocoagulation (PRP) can stabilize or reverse neovascularization in the retina. A two-year trial is currently evaluating the safety and efficacy of prompt vitrectomy plus PRP vs. aflibercept injections in the treatment of eyes with VH from PDR.13
Keep in mind that intravitreal injections of anti-VEGF do not come without complications, which must be taken into account before pursuing this option as your treatment of choice.14
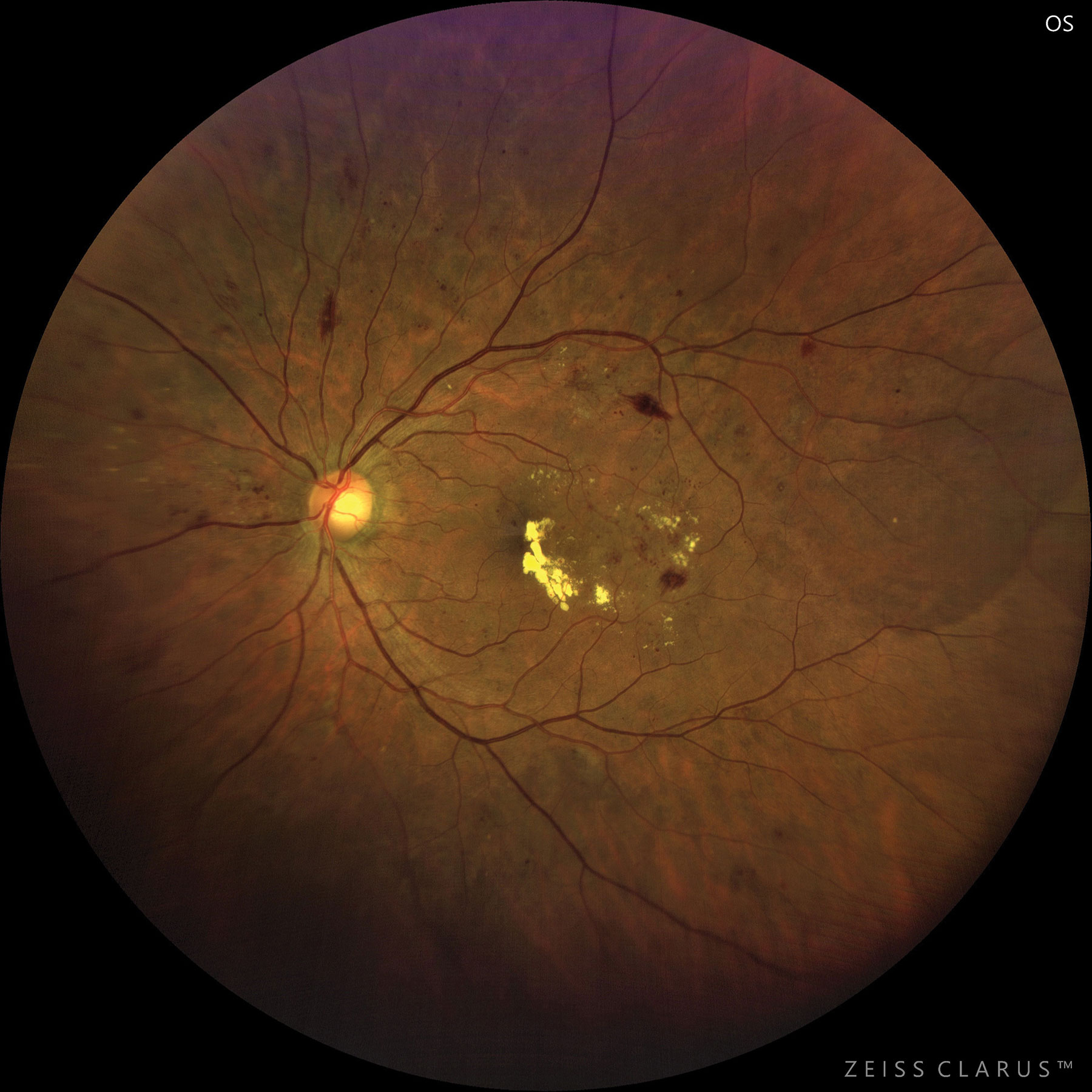 |
| This patient with moderate to severe NPDR developed CSME. Photo: Jay M. Haynie, OD. Click image to enlarge. |
Managing NPDR
Between 12% and 27% of patients with moderate NPDR without DME progress to PDR in one year.1 Moderate NPDR care consists of managing blood pressure, blood glucose level and other risk factors (blood lipids, cardiovascular risk, physical health, weight) and following up every six to nine months. Currently, there is no treatment for moderate NPDR.
PANORAMA, a Phase III trial, evaluated whether aflibercept can prevent the progression of moderate to severe NPDR—as determined by the Diabetic Retinopathy Severity Scale (DRSS) score—thereby helping reduce the incidence of DME.15,16 The randomized, double-masked trial included one control group and two aflibercept treatment groups—134 patients received aflibercept every eight weeks and 135 received it every 16 weeks.15
After two years, 61.5% of participants treated every 16 weeks and 55.2% of participants treated every eight weeks experienced at least a two-step improvement from baseline in DRSS score.15 In the control group, on the other hand, only 6.0% of the 133 participants experienced a two-step or greater improvement.15
Regarding adverse events, 3.7% of patients treated every 16 weeks and 5.2% of patients treated every eight weeks experienced a vision-threatening complication or CI DME compared with 25.6% of controls.15 The trial indicated that “consistent aflibercept treatment significantly prevented vision-threatening complications and improved DRSS score on moderately severe to severe NPDR.”17
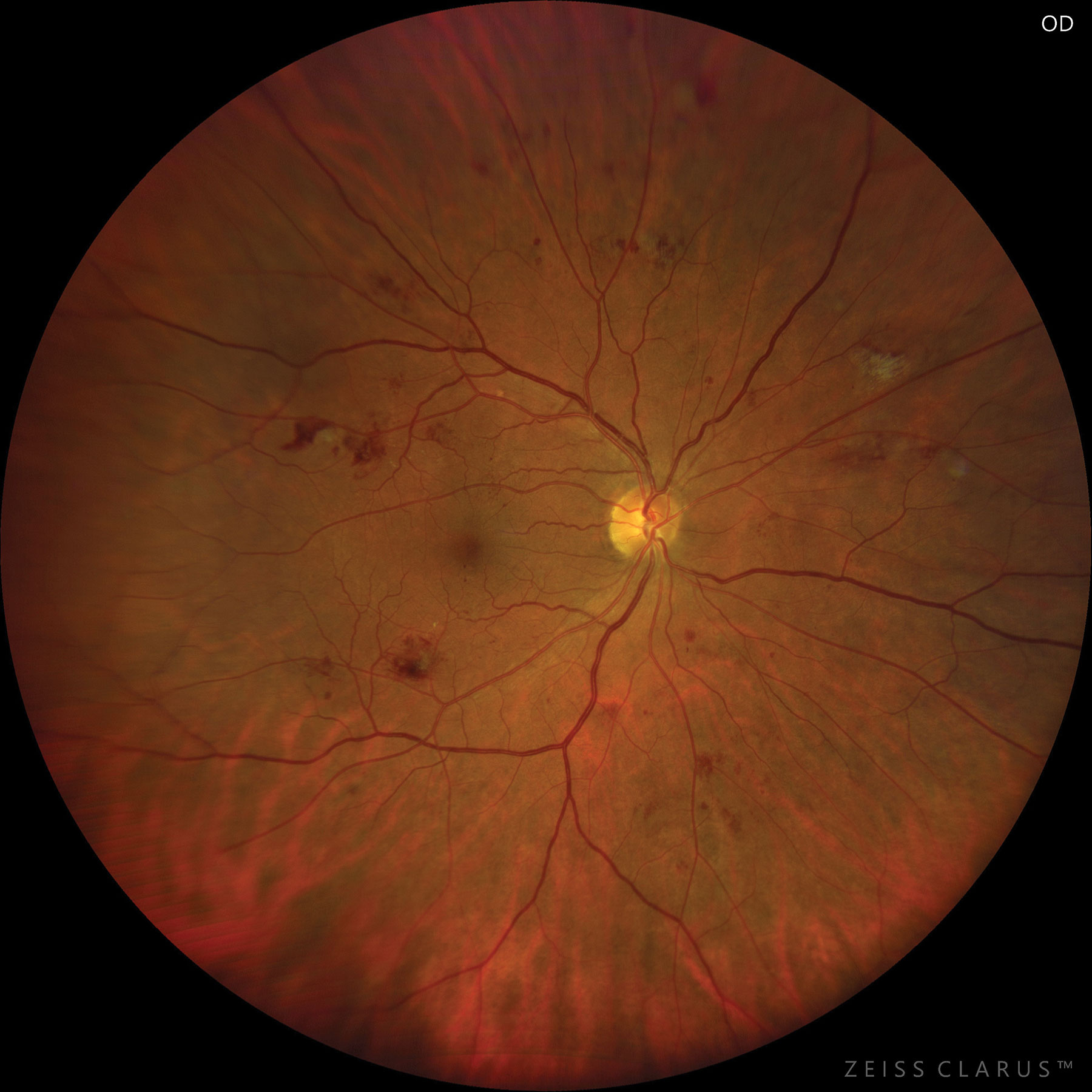
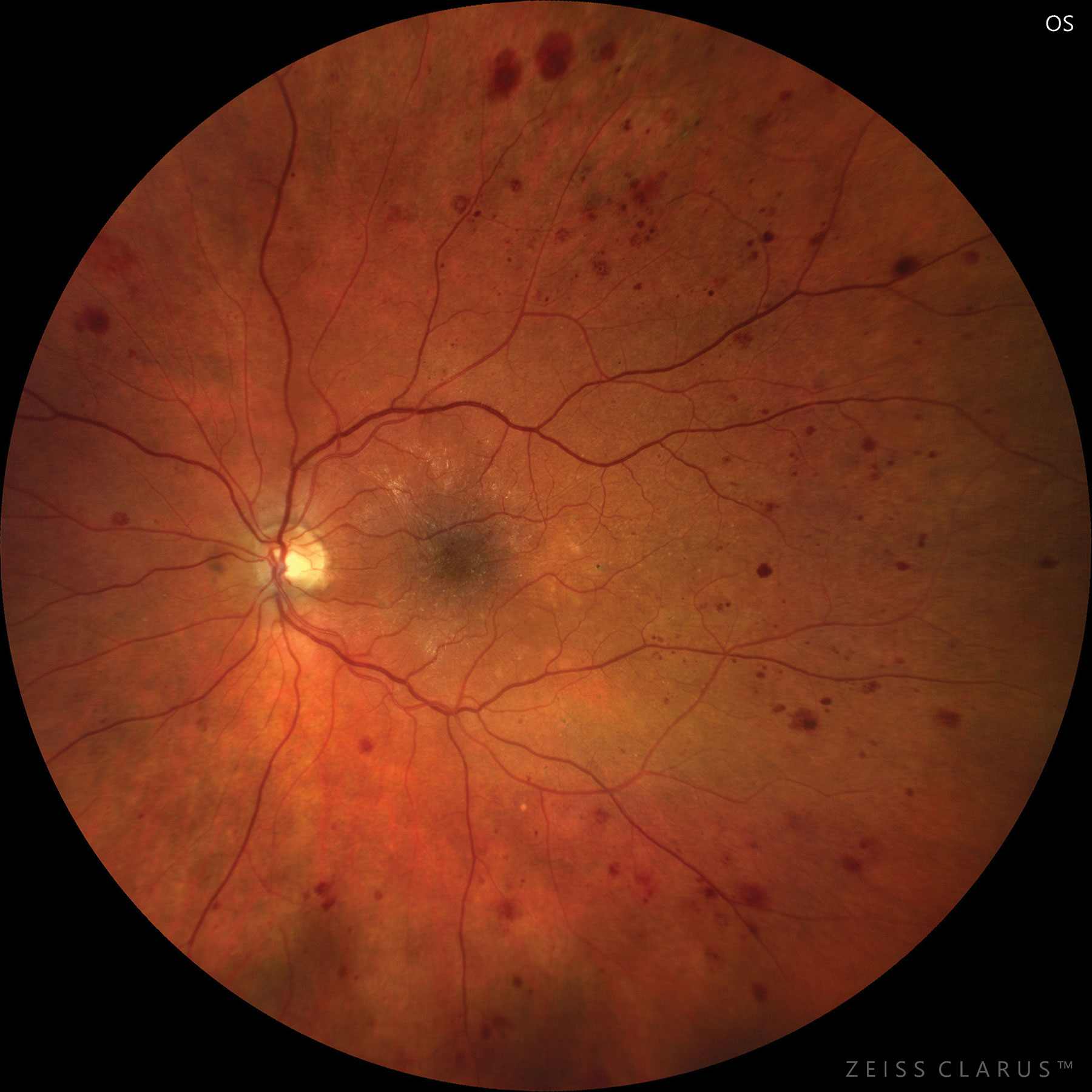 |
| Severe NPDR based on hemorrhages and microaneurysms in four retinal quadrants. Photo: Jay M. Haynie, OD. Click image to enlarge. |
Managing PDR
PRP, first proposed in the 1960s, has been the standard of care for treating PDR by coagulation of areas of capillary non-perfusion marked by fluorescein angiography, thereby preventing the retinal blood vessels from leaking. However, PRP can sometimes be limited in its ability to reduce neovascularization, and it destroys photoreceptors—especially rods—so vision and peripheral fields rarely improve after PRP treatment. VEGF is a major causative factor in the neovascularization seen in PDR and the vascular permeability in DME, raising the question of whether anti-VEGF can prevent PDR progression and DME.10
A pair of studies—DRCR’s Protocol S—showed that ranibizumab therapy was non-inferior to PRP on visual acuity measurement at two and five years for PDR treatment.18,19 Fewer patients treated with ranibizumab developed DME and needed vitrectomy surgery, and there was less visual field loss in the group receiving therapy.18,19 Unfortunately, it is unclear how long the retinal neovascularization regression will last after the cessation of anti-VEGF treatment.
An ongoing trial is currently evaluating whether aflibercept injections prevent PDR progression or DME in high-risk patients.20 The team is monitoring visual acuity measurements, OCT findings and the ratio of eyes with at least a two-step worsening to those with at least a two-step improvement in DRSS score over a period of two years.20 It is possible that anti-VEGF treatment can delay or prevent the need for PRP and reduce the frequency of intravitreal injections for DME.
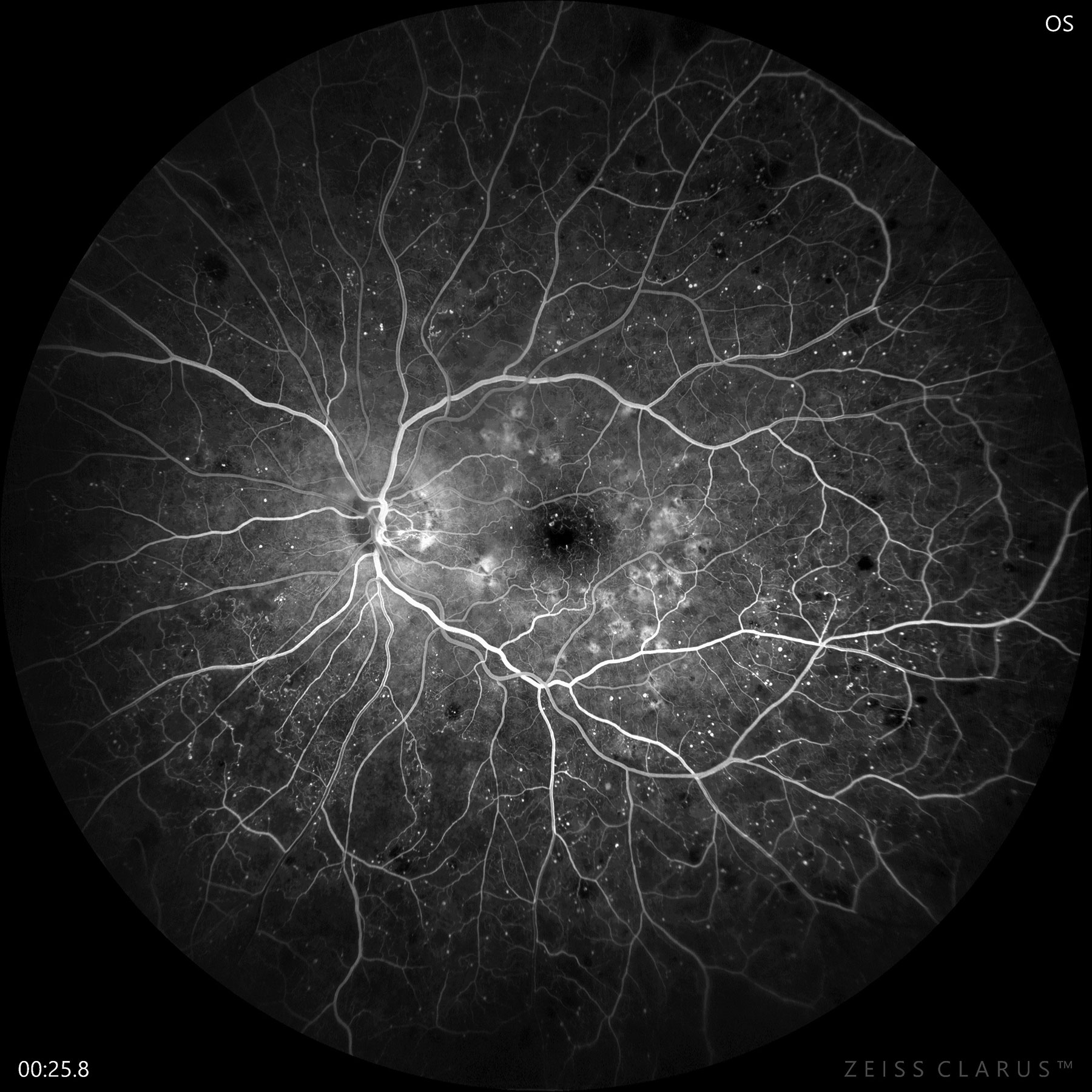 |
| Fluorescein angiography confirms early PDR with significant non-perfusion and neovascularization of the disc. Photo: Jay M. Haynie, OD. Click image to enlarge. |
Step-by-step Approach
Diabetes management differs from office to office, with no consistent standard of care, and is constantly evolving with advances in treatment. Taking into account findings that have emerged from recent studies and my experience as an optometrist, I recommend the following when caring for patients with this condition:
1. Screen. Testing for DR is the primary task of an ophthalmic examination for patients with diabetes and the first step toward early detection. Many primary care providers (PCPs) refer their patients with diabetes to an optometrist for a dilated fundus exam. Regardless of the diagnosis, this presents a good opportunity for the optometrist to build a relationship with the PCP, as it is likely that they will be working alongside each other to provide the best joint care for the patient.
2. Document. Upon detecting DR, the optometrist should record disease severity and whether DME or CSME is present. Keep a schedule of your treatment or referral and follow-up plans with a clearly-marked timeframe. Detailed charting provides a clear picture of the patient’s journey, a consistent means of reporting and protection from potential malpractice lawsuits.
3. Educate. Keeping patients informed may be a lot of work upfront but pays off later on. Topics should include risk factors of DR, what effects they can have and how to manage them. Management includes glycemic control, smoking cessation, blood pressure monitoring, lipid-lowering treatment, cardiovascular risk reduction, physical exercise and weight management.1 Have pamphlets on diabetes and DR available in your office, and be ready to show your patients their scan results so they can better visualize their condition. Patient education has mutual benefits, as it facilitates understanding and promotes compliance in addition to reducing the chance of DR progression and establishing a long-term doctor-patient relationship.
4. Refer. Following the published guidelines from the AOA, refer patients with severe NPDR, PDR or DME to an ophthalmologist.1 These patients stand to benefit from anti-VEGF treatment, as supported by recent findings. In addition, I recommend referring cases of moderate NPDR without DME but with high A1c levels. Low vision specialists can also help improve the quality of vision and life for severe PDR and DME patients.
5. Monitor. Whether you choose to refer or not, it is important to follow up with your DR patients so you can manage their condition and intervene if necessary. In the event of a referral, the patient is still your responsibility. Research shows that comanagement between local optometrists and hospital-based ophthalmologists improves care for patients with DR.21
Telehealth and DROne of the most popular telemedicine applications for DR screening is teleretinal imaging. The program currently assesses veterans with diabetes and is especially useful in rural parts of the country where fewer eye doctors are available. The teleretinal imaging process is simple and convenient. A nurse or technician takes retinal photos of a diabetes patient with a fundus camera. The photos are then sent to the teleretinal imaging reading center, where an optometrist reviews them. The diagnosis and, if positive for DR, severity of DR and recommendation are saved and sent back to the nurse or technician. The program has specificity and sensitivity values of 95% and 86%, respectively. Exclusion criteria include patients who have had retinal laser treatment, retinal surgery or intravitreal injection and those who are monocular. The follow-up schedule looks very similar to that associated with a dilated fundus exam for DR. If retinal photos show PDR, severe NPDR or probable CSME, a face-to-face appointment with an eye care provider is required. In-person evaluations are recommended if retinal photos are blurry, DME, AMD or glaucoma is suspected or a choroidal scar is detected. Most patients’ attitudes toward teleretinal screening are positive, as this process is faster, easier, more accessible and more convenient than traditional in-office screening. It also reduces travel time and cost. On the other hand, the equipment and necessary training is expensive and images may not always be gradable due to poor visual fields, media opacities or small pupil sizes. Dilated pupils also increase the risk of acute angle-closure glaucoma. All in all, teleretinal imaging represents a substantial step forward for optometry and the care we’re able to offer our patients with diabetes.
|
Recent clinical trials have introduced the idea of proactive anti-VEGF treatment for DR to prevent vision-threatening complications and progression from moderate to severe cases. Armed with this new knowledge, optometrists can now play a more important role than ever in providing the most comprehensive care for patients with diabetes.
Dr. Yuen practices at the Central Texas VA Health Care System in Temple, TX, and is a fellow of the American Academy of Optometry.
| 1. AOA. Evidence-based Clinical Practice Guideline: Eye Care of the Patient with Diabetes Mellitus. aoa.uberflip.com/i/1183026-evidence-based-clinical-practice-guideline-eye-care-of-the-patient-with-diabetes-mellitus-second-edition/0?m4=. October 4, 2019. Accessed April 29, 2020. 2. Hein TW, Potts LB, Xu W, et al. Temporal development of retinal arteriolar endothelial dysfunction in porcine type 1 diabetes. Invest Ophthalmol Vis Sci. 2012;53(13):7943-9. 3. Murakami T, Nishijima K, Akagi T, et al. Segmentational analysis of retinal thickness after vitrectomy in diabetic macular edema. Invest Ophthalmol Vis Sci. 2012;53(10):6668-74. 4. Carpineto P, Mastropasqua R, Marchini G, et al. Reproducibility and repeatability of foveal avascular zone measurements in healthy subjects by optical coherence tomography angiography. Br J Ophthalmol. 2016;100(5):671-6. 5. ClinicalTrials.gov. Intravitreal aflibercept for retinal non-perfusion in proliferative diabetic retinopathy (RECOVERY). clinicaltrials.gov/ct2/show/NCT02863354. August 11, 2016. Accessed April 29, 2020. 6. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796-806. 7. Stefanini FR, Arevalo JF, Maia M. Bevacizumab for the management of diabetic macular edema. World J Diabetes. 2013;4(2):19-26. 8. Lechner J, O’Leary OE, Stitt AW. The pathology associated with diabetic retinopathy. Vision Res. 2017;139:7-14. 9. Grant MB, Afzal A, Spoerri P, et al. The role of growth factors in the pathogenesis of diabetic retinopathy. Expert Opin Investig Drugs. 2004;13(10):1275-93. 10. Gupta N, Mansoor S, Sharma A, et al. Diabetic retinopathy and VEGF. Open Ophthalmol J. 2013;7:4-10. 11. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801. 12. Fong AH, Lai TY. Long-term effectiveness of ranibizumab for age-related macular degeneration and diabetic macular edema. Clin Interv Aging. 2013;8:467-83. 13. ClinicalTrials.gov. Anti-VEGF vs. prompt vitrectomy for VH from PDR (AB). clinicaltrials.gov/ct2/show/NCT02858076. August 8, 2016. Accessed April 29, 2020. 14. Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond). 2013;27(7):787-94. 15. ClinicalTrials.gov. Study of the efficacy and safety of intravitreal (IVT) aflibercept for the improvement of moderately severe to severe nonproliferative diabetic retinopathy (NPDR) (PANORAMA). clinicaltrials.gov/ct2/show/results/NCT02718326?cond=diabetic+Aflibercept&draw=2&rank=18. March 24, 2016. Accessed April 29, 2020. 16. Staurenghi G, Feltgen N, Arnold JJ, et al. Impact of baseline diabetic retinopathy severity scale scores on visual outcomes in the VIVID-DME and VISTA-DME studies. Br J Ophthalmol. 2018;102(7):954-8. 17. Wykoff CC. A phase 3, double-masked, randomized study of the efficacy and safety of aflibercept in patients with moderately severe to severe NPDR. Presented at: Angiogenesis, Exudation, and Degeneration 2020; Feb. 8, 2020; Miami. 18. Gross JG, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs. intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137-46. 19. Gross JG, Glassman AR, Liu D, et al. Five-year outcomes of panretinal photocoagulation vs. intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138-48. 20. ClinicalTrials.gov. Anti-VEGF treatment for prevention of PDR/DME. clinicaltrials.gov/ct2/show/NCT02634333. December 18, 2015. Accessed April 29, 2020. 21. O’Connor PM, Harper CA, Brunton CL, et al. Shared care for chronic eye diseases: perspectives of ophthalmologists, optometrists and patients. Med J Aust. 2012;196(10):646-50. |

