Transformative TechOptometrists today have more tools than ever at their disposal to help detect, treat and manage disease. To help you keep up with the constant innovation, the September 2024 issue of Review of Optometry—our 47th annual technology report—reviews the pros and cons of telehealth in optometry, new devices in eye care and the future of remote monitoring. Plus, find the results of our annual tech survey to discover which clinical instruments your peers are buying or desiring. Check out the other articles featured in this issue: |
Healthcare is not experienced equitably by all populations. Rural communities in particular face barriers to care that urban and suburban populations do not. These barriers are multifaceted and include geographic, technological and economic factors. Any approach to mitigation must itself be multifaceted, since addressing only one will not overcome the effects of the other dynamics at play that limit access to care.
Technological innovations have led to a boom in digital health, a network of systems that allows patients to interact with their provider via digital interfaces. Importantly, digital health tools are adjunctive technologies, not ones to be used in lieu of seeing a clinician in office. That is, they allow practices to extend and deepen patient relationships but do not replace providers.
In eye care, services such as autonomous diabetic retinopathy severity grading, home tonometry for patients with glaucoma and home monitoring for age-related macular degeneration (AMD) progression empower providers to keep patients in rural areas or those with other access limitations within their orbit of care. These services also help providers in regions that straddle the rural-suburban divide to keep patients at risk of loss to follow-up on the right side of the care spectrum.
We, the authors, have direct experience in treating such populations. One of us (CM) practices in Tahlequah, OK, where the patient base is largely Native American. These individuals face barriers to care linked to their rural status, among other factors such as historic marginalization. The other (AL) practices in Wyomissing, PA, a small suburban metro nestled between suburban population centers to its south, east and west, and the more rural Appalachian Mountains to its north.
Given our experience with rural populations who face barriers to care, we hope to update our optometric colleagues about the latest data surrounding rural health disparities and explore how digital health tools could better serve our patients and improve our practices.
 |
|
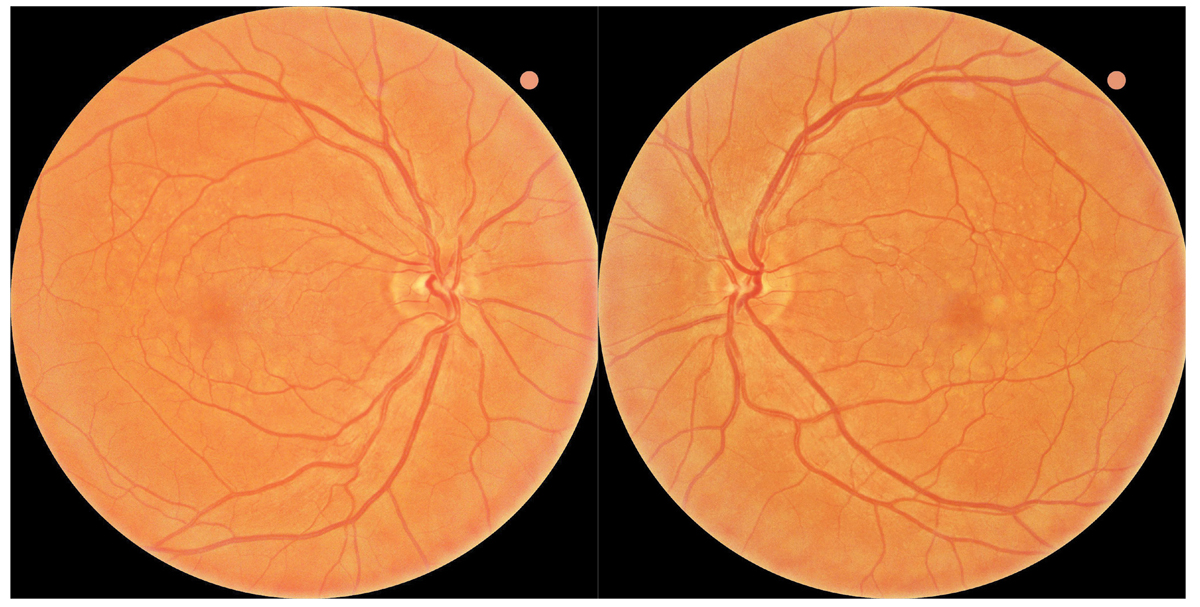
Fig. 1. Baseline fundus photography revealed large soft drusen bilaterally consistent with intermediate stage AMD. The patient was referred to the ForeseeHome AMD Monitoring Program and instructed to follow up in six months. Click image to enlarge. |
The State of Play in Rural Health Care
One of the most obvious challenges to accessing care for rural inhabitants is the distance from the patient’s home to the clinic. A review of cardiovascular clinical trial sites, which typically enroll in larger population centers, found that only 5% of sites were in rural areas.1 The median distance between a clinical trial site and the patient’s home was 5.8km; this distance was 4.5km for urban patients and 24.2km for rural patients. It stands to reason that when patients cannot dedicate the time and financial costs of longer travel, they lose access to care (whether from conventional clinical visits or clinical trial enrollment). Distance to the clinic may be a uniquely intense barrier to care for patients with vision-related issues, as they often require a caregiver to be available to drive them to their appointment, which means that two people (rather than just the patient) need to be available for an appointment.
In eye care, the differences in access to care have led to higher rates of blindness in rural areas compared with non-rural areas.2 A 2023 retrospective analysis of the IRIS Registry from the American Academy of Ophthalmology found that blindness rates were positively associated with patients in rural settings. Blindness rates were also positively associated with having public or no insurance, underscoring the link between low income and worse outcomes.
Rates of ophthalmic care are worse among Native American and Alaska Native populations. A recent retrospective, cross-sectional study of peer-reviewed literature from the past 25 years found that retinopathy, cataract, vision impairment and blindness were higher among these populations than other American population cohorts.3 Although rates of macular degeneration and glaucoma were similar among all patient populations, treatment rates were lower among Native populations, which led to poorer outcomes in those groups.
Zooming out to focus on healthcare disparities in general for rural populations can help us grasp the dynamics at play. One study that focused on American providers’ perspectives on the barriers to care concluded that costs, insurance-related issues, geographic dispersion and provider shortage/burnout were some of the chief issues facing rural populations.4 These providers suggested that greater use of telehealth services and establishment of mobile clinics for specialty care could be key to improving access to health in rural communities.
Still, implementation of, say, a fleet of mobile clinics would require expensive and time-consuming projects that local specialists may be ill-equipped to initiate or uninterested in undertaking. Home-based telehealth tools—with little to no capital overhead, physical clinical footprint or changes to practice workflow—could give patients access to care that they otherwise may have found too difficult to acquire.
 |
|
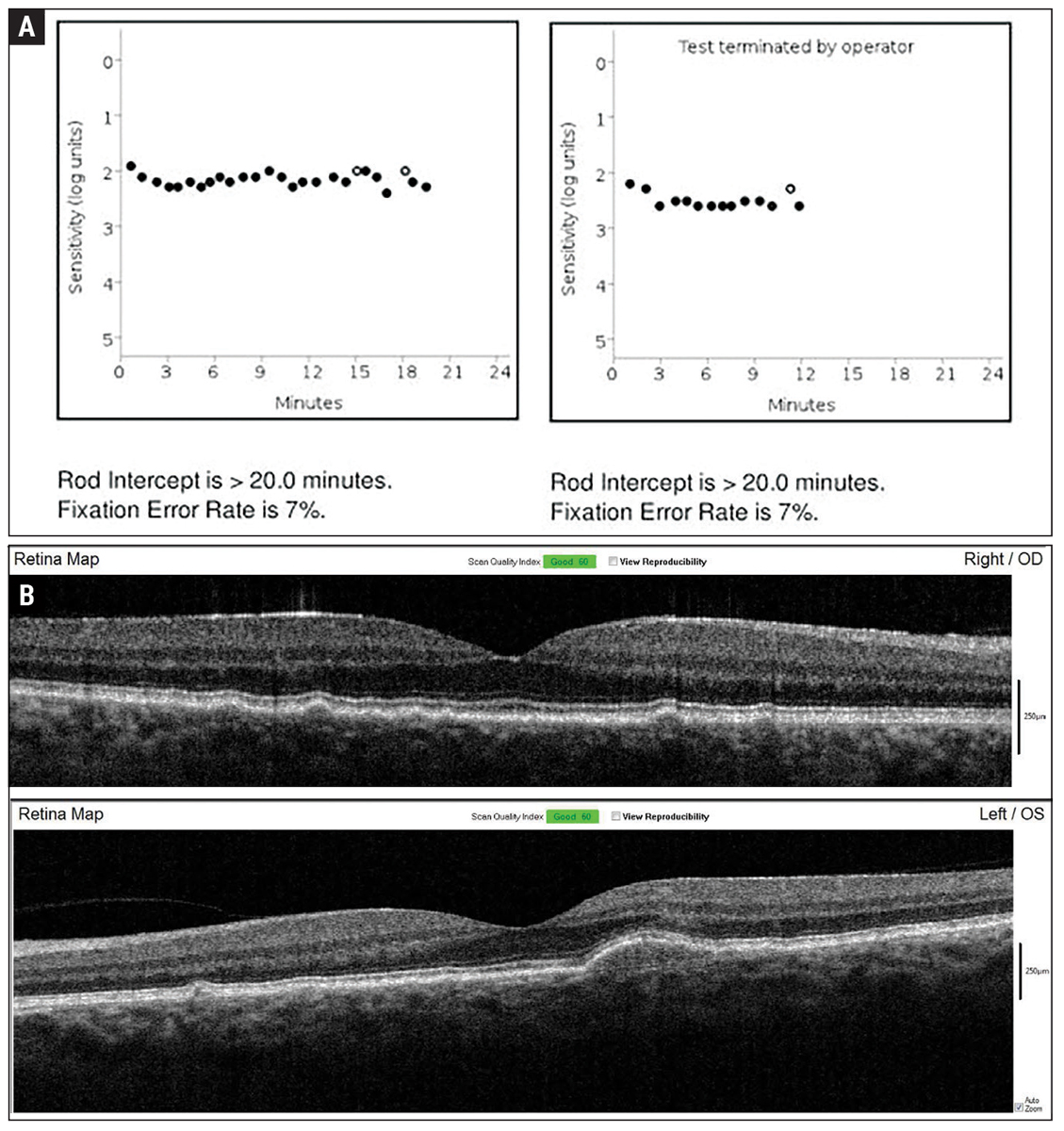
Fig. 2. Baseline dark adaptation (A) and OCT imaging (B) were established in-office upon initiation of home monitoring for wet exudative AMD conversion. Click image to enlarge. |
The Rise of Remote Monitoring
Outside of eye care, researchers have examined the utility of remote monitoring to detect atrial fibrillation via an Apple Watch.5 Approximately 419,000 patients with no self-reported history of atrial fibrillation wore an Apple Watch for a median 117 days. Patients were notified if irregular pulse was detected, at which point an electrocardiography (ECG) patch was mailed to them with instructions to wear it for seven days. Among all patients, 0.52% received notifications of irregular pulse, and among those who returned the ECG patch for analysis, approximately 35% of patients were confirmed to have atrial fibrillation. Interestingly, no site visits were required in this study, as all testing and communications were performed at home. This illustrates the power of remote monitoring to effectively trigger further scrutiny of a patient.
There are benefits to embracing innovative technology in eye care. Using AI-driven technology in eye care for rural patient populations has been effective at increasing examination rates. A study published in 2024 assessed the completion rate of diabetic eye exams among pediatric patients with type 1 or type 2 diabetes in rural settings.6 Patients were randomly assigned to an intervention arm (wherein an autonomous AI diabetic eye exam was used at the point of care in an academic pediatric diabetes center; n=81) or a control arm (wherein the patient received scripted eyecare provider referral and education; n=83). At six months, the researchers found that 100% of patients in the intervention arm had completed a diabetic eye exam compared with 22% of controls. Furthermore, of those in the interventional arm whose results indicated that follow-up was needed (25 of 81 patients), 64% completed a follow-up appointment with an eyecare provider. This was significantly higher than the 22% of patients in the control arm who followed up with an eyecare provider.
At-home Monitoring in Glaucoma
Three of the modalities often used in the care of glaucoma patients are potentially conducive to remote data capture. If these modalities—non-mydriatic fundus photography, visual field testing and tonometry—were to become more widely used in-home or in remote settings, providers dedicated to glaucoma care in primary or specialty settings may be able to harvest accurate data more frequently, thereby better informing treatment strategies.
The iCare Home and iCare Home2 (iCare) are home-based tonometers that track diurnal intraocular pressure (IOP). They provide longitudinal data that supplement those captured in the clinic. A pivotal study assessing the iCare Home’s efficacy showed that it detected therapy-related changes in IOP, and further research has found it useful in monitoring peri-interventional patterns.7,8 Agreement between the home tonometers and the office-based Goldmann tonometry has been shown to be within 5mm Hg in 91% of cases, with a mean difference of 0.33mm Hg, suggesting that the two devices may be similar enough to render differences negligible for some patients.9 Perhaps most importantly, patients like it: 89% of those who have used the iCare Home said they would recommend it to other patients with glaucoma.10
Optometrists can purchase the iCare Home or the iCare Home2 and charge patients for at-home use. Alternatively, they can write a prescription for the device, permitting the patient to either purchase or rent the device from a third party. Doctors receive patient results either through a web portal (if they have loaned out the device) or via a report sent from the third party (if the patient has received a prescription and has rented or purchased the device for themselves). Long-term glaucoma patients in the care of optometrists seeking to increase the frequency of IOP monitoring may be well-suited for these devices. In particular, home tonometry may provide valuable insight for patients with glaucomatous progression despite seemingly well-controlled IOP measures in office.
Use of smartphone-based non-mydriatic fundus photography to measure cup-to-disc ratio of the optic nerve head has been shown effective in assessing at-risk patients. A 2021 study assessing the ability of PanOptic iExaminer (Welch Allyn), an apparatus that attaches to an iPhone camera, concluded that use of a such a device was an effective, low-cost means of screening patients for glaucoma risk. Of the 206 patients enrolled in the study, 16% had characteristics suggestive of glaucoma; these patients were referred for subspecialist evaluation, 94% of whom met the criteria for potential glaucoma.11
The study in question took place in Samoa, which, per the study authors, is “an underserved setting with one full-time ophthalmologist in the entire country”—precisely the type of rural setting that we feel could maximally benefit from remote data capture.11
The VisuAll Virtual Reality Platform (VRP; Olleyes) is a headset device that can be used in remote (i.e., home) settings to perform visual field testing. A 2024 study assessing the feasibility, accuracy and repeatability of home-based visual field testing enrolled 15 participants, nine of whom completed the study; the six patients who did not complete the study had difficulty acquiring home-based tonometry data, which was a requirement for study completion.
During a session in which patients were trained to use the VRP, patients sat for a Humphrey Field Analyzer (HFA) visual field test, and the results of VRP testing were compared to HFA assessments.12
The results were encouraging. After three consecutive days of home testing, the researchers observed significant correlation between the average mean deviation values of VRP and HFA testing and found that the time to capture VRP data was significantly shorter compared to HFA. Five of the six Garway-Heath sectors on the visual field were significantly correlated between VRP and HFA.
Providers need deeper datasets to understand if home-based visual field data capture is reliable, but this early feasibility study reflects positively on the concept of home-based visual field data capture. Patients with glaucoma living in remote settings may benefit from this technology if, for instance, their provider instructs them to visit the clinic less frequently than in the days when clinic-based assessments were key to patient care.
 |
|
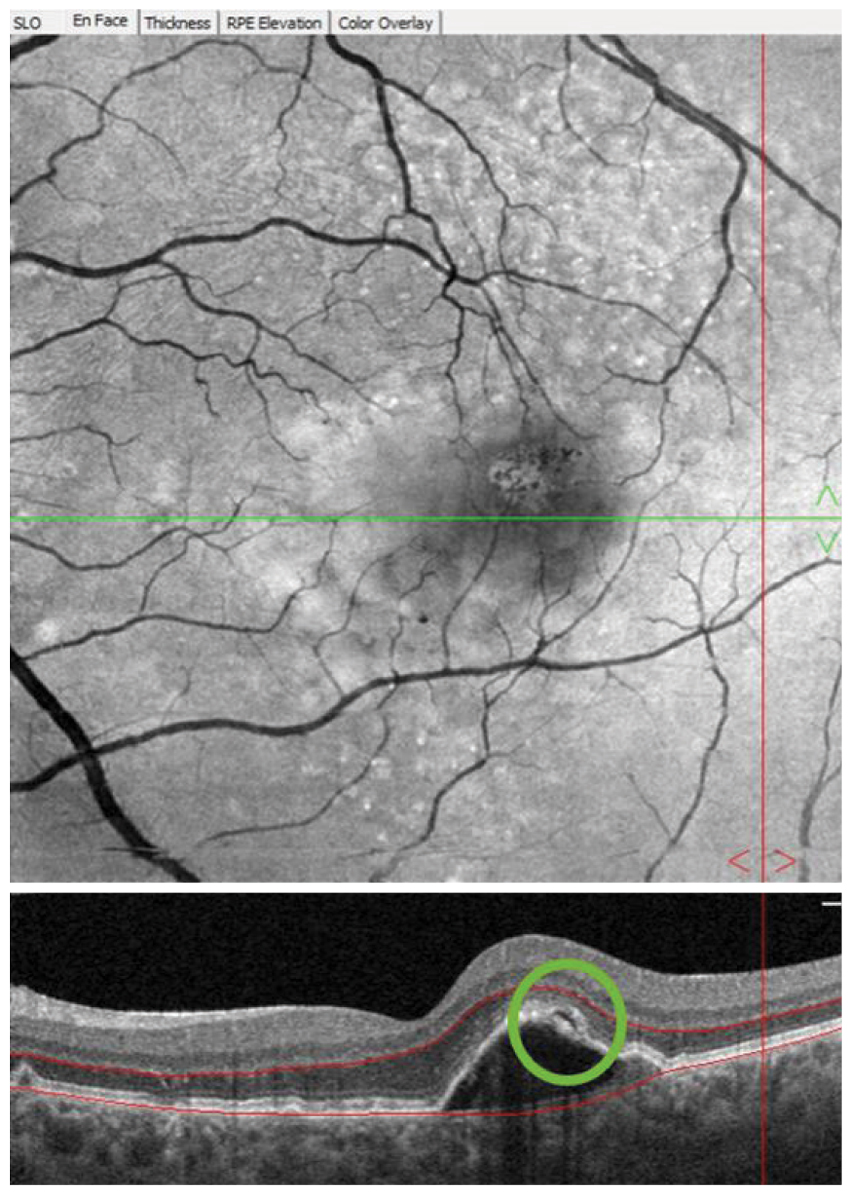
Fig. 3. Following a device alert three years into home monitoring, the patient returned to the optometry clinic for examination. A pigment epithelial detachment with overlying subretinal fluid (green circle) was observed on OCT imaging and anti-VEGF therapy was initiated upon retinal consultation. Click image to enlarge. |
Digital Remote Monitoring and Quality of Care
Options that can help detect the progression from intermediate AMD to advanced wet AMD include mobile applications and a dedicated device. Apps that we have heard mentioned by our colleagues, but we have not used, include myVision Track and Macustat. Mobile applications are easy for patients to access via their smart devices or computers and represent an important development in the digital remote monitoring landscape. Still, although reports have appeared in the literature assessing these technologies, they have not been validated in randomized controlled trials.13-15
One such technology that both uses AI and has been proven effective in a randomized controlled trial is the ForeseeHome (Notal Vision).16 This technology marries two approaches outlined above: a dedicated home-based device (outlined in the Apple Watch study) that is driven by AI (as seen in the pediatric diabetic eye disease study), giving patients the chance to embrace a remote monitoring solution with an AI foundation.
First, a primer on ForeseeHome, which is used to assist the physician in detecting conversion from intermediate to advanced wet AMD. Per the Beckman Classification, patients are subtyped into having early, intermediate or advanced AMD; patients with advanced disease are subtyped into wet AMD or geographic atrophy (GA), which are not mutually exclusive.17 Treatments for both wet AMD and GA are approved for use; still, close monitoring for intermediate AMD patients is advised, as early detection of conversion to advanced AMD can lead to early intervention which is associated with improved anatomic and visual outcomes.
Providers prescribe ForeseeHome, which measures hyperacuity, to patients with intermediate AMD to help in the early detection of wet AMD; hyperacuity is impacted earlier in AMD than conventional acuity. Patients set up the device in their homes and perform regular testing between routine eye examination visits. Data is collected by a monitoring center operated by the manufacturer. If the software detects an aberration that may signal conversion from intermediate to advanced AMD, the prescribing provider’s office is notified so that they can reach out to the patient to schedule an exam. If the device has not been used for an extended period, the patient is contacted and reminded by the monitoring center to use the device; any troubleshooting that the patient needs occurs at this time.
In the pivotal HOME study, researchers concluded the device was effective at detecting choroidal neovascularization (CNV) and increased “the likelihood of better visual acuity results after intravitreal anti-VEGF therapy.”18 Real-world studies have shown that use of ForeseeHome led to early detection of wet AMD conversion, and that median visual acuity at baseline, conversion and final follow-up for eyes that converted during the study’s monitoring period were 20/30, 20/39 and 20/32, respectively.19
For rural populations, remote monitoring of conversion to wet AMD could be particularly beneficial. Patients will still be expected to present for in-office eye exams but will also be kept in the practice’s orbit via remote monitoring. Rural patients (as well as older patients who have difficulty making it into the clinic) may find that remote monitoring engenders a sense of closeness to their provider and deepens their relationship to care. After all, testing several times per week keeps ocular health top of mind for the patient, and knowing that your provider’s office will contact you if the device triggers an alert ensures that your provider won’t let you slip through the cracks. These benefits mitigate the challenges rural patients face and “reduce the distance” between the patient’s home and a specialist’s clinic.
False Positives and Potential Challenges
Patients and providers may be understandably worried that use of remote devices may trigger an alert of possible conversion to wet AMD that is, upon in-person clinical assessment, not observed. Such a visit could be a waste of time and money; however, false positives (FPs) still hold value. A subanalysis of the ALOFT study found that eyes that had triggered an FP on the ForeseeHome were at higher risk for conversion to wet AMD than eyes that hadn’t.20 One study speculated that FPs could be due to the non-observable changes to retinal tissue that affect the functional hyperacuity test score measured by the device.20
In this sense, at-home remote monitoring still informs patients and providers even in the event of an FP. If such an event were triggered, a provider may direct the patient to come in more frequently such that any clinically observable conversions to wet AMD could be detected via examination.
This is not to say, of course, that home-based monitoring is not without its limits. Two fundamental facts may be at loggerheads: reliable digital connectivity is fundamental to capturing data with a device such as the ForeseeHome, and patients in rural settings may struggle to access a high-quality connection. Patients with limited tech literacy may find remote monitoring platforms intimidating or, even if they find themselves using such a platform, too difficult to troubleshoot even with the direct-to-patient assistance offered by the Notal Vision Monitoring Center. Even though the program is covered by Medicare, some patients may nevertheless be turned off by the idea of service that could be (however erroneously the conception) associated with a recurring fee.
 |
|
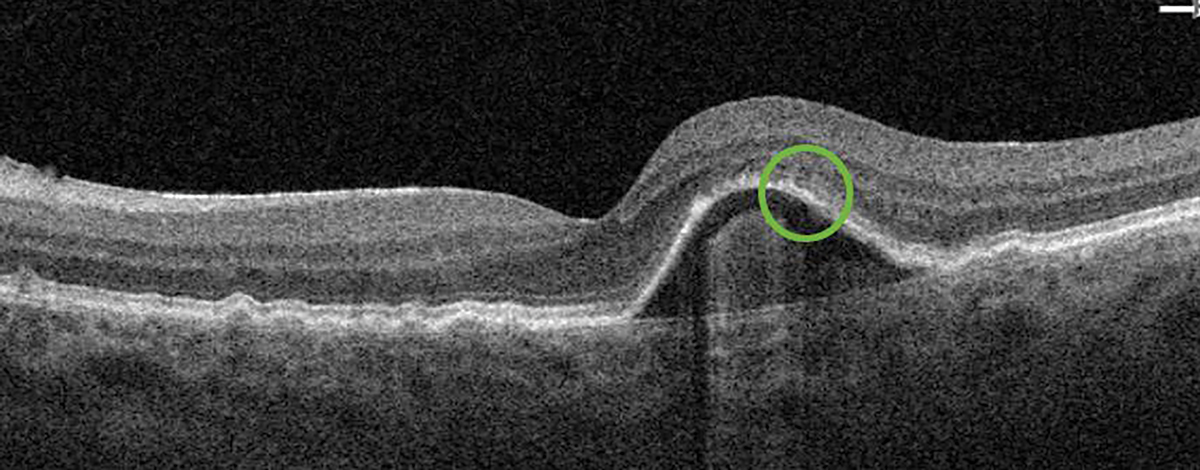
Fig. 4. The subretinal fluid (previously in the green circle) observed during the first post-alert examination resolved after the initiation of anti-VEGF therapy. Click image to enlarge. |
Real-World Case
A 55-year-old Caucasian man presented to the Wyomissing Optometric Center for baseline retinal exam with uncorrected acuity of 20/20 in each eye. Ophthalmoscopy revealed large soft drusen, and the patient was diagnosed with intermediate stage nonexudative AMD bilaterally (Figure 1). Baseline baseline dark adaptation and OCT were obtained in-office (Figure 2).
AREDS2 supplementation was recommended, and the patient was referred to the ForeseeHome AMD Monitoring Program and began home surveillance. He was asked to return to clinic in six months.
Almost three years later, a ForeseeHome alert OS was generated, and the patient was brought in to the office for examination that same day. OCT showed a pigment epithelial detachment with overlying subretinal fluid OS suspicious for new-onset advanced wet AMD (Figure 3). Uncorrected VA of 20/20 OS was measured at this visit and the patient was referred to a retina specialist for further evaluation and treatment. Intravenous fluorescein angiography performed by the retina specialist revealed late leakage OS, and anti-VEGF therapy was initiated. At the six-week post-injection follow-up, excellent uncorrected visual acuity of 20/20 OS was maintained, which may be, in part, due to early detection of wet AMD before symptoms developed.
Future of Home-Based Care in Retina and Glaucoma
The prospects for remote monitoring in eye care will likely include use of home-based OCT imaging for monitoring retinal tissue. One such device, the Scanly Home OCT (Notal Vision), was recently granted FDA de novo marketing authorization. It will allow treating physicians to garner never-before-seen disease insights of wet AMD activity by capturing and collating data in the time between office visits.
The latest data on home OCT imaging show that patients are comfortable and competent at acquiring usable OCT images.21 The quality of home OCT scans is comparable to those obtained by in-office OCT. Pivotal trials specifically compared visualization of key biomarkers for wet AMD in home OCT and in-office OCT, demonstrating an equivalence between the two.22 Similar results were found in DRCR Retina Network’s clinical study, Protocol AK, where investigators found strong agreement in presence of fluid between home OCT and in-office OCT.23 When optometric physicians familiarize patients with remote monitoring protocols and technology before the patient converts to wet AMD, in addition to better long-term visual outcomes from early detection, patients may be more likely to embrace the home OCT monitoring during treatment.19
Patients in rural settings who face difficulties with follow-up adherence to retina specialists’ appointments may be particularly drawn to Scanly Home OCT, as it could lessen the frequency with which they need to visit a retinal clinic for care. Reduced treatment burden for wet AMD could aid in reducing the disparities between rural and non-urban populations, as well as the disparities between low-income and wealthier patients.
Takeaways
Optometric physicians play a unique social role for communities that have faced barriers to health care access. Embracing remote monitoring may play a role in closing the disparity gap in these areas. It falls on us primary eyecare providers to make sure that the right patients are prescribed, trained on and educated on these technologies that could eventually be available for them to take advantage of. Timely delivery of care may depend on it.
Dr. Legge practices at the Wyomissing Optometric Center in Wyomissing, PA. She serves as a member of the Allied Health Professional Staff at Penn State Health St. Joseph Medical Center (inpatient consults and emergency department eye care). She is a consultant and speaker for LKC Technologies, and has previously held these roles for MacuLogix, MacuHealth, Notal Vision and Astellas Pharma.
Dr. Majcher is a professor and the director of residency programs at the Northeastern State University Oklahoma College of Optometry, as well as a fellow of the American Academy of Optometry and the Optometric Retina Society. She received her doctorate in optometry from the Pennsylvania College of Optometry at Salus University and completed an ocular disease residency at the Eye Institute of the Pennsylvania College of Optometry. She is a paid speaker and consultant for Regeneron, Carl Zeiss Meditec, Apellis and Astellas. She is also a paid consultant for Topcon, Notal Vision, Lenz Therapeutics and Tarsus and has received non-financial support from Roche.
1. Salerno PRVO, Bourges-Sevenier B, Chen Z, et al. Geographic proximity to cardiovascular clinical trial sites: a national analysis in the United States. Curr Probl Cardiol. 2024;49(8):102683. 2. Brant A, Kolomeyer N, Goldberg JL, et al. United States population disparities in ophthalmic care: blindness and visual impairment in the IRIS Registry (Intelligent Research in Sight). Ophthalmology. 2023;130(11):1121-37. 3. Miller AM, Gill MK. A review of the prevalence of ophthalmologic diseases in Native American populations. Am J Ophthalmol. 2023;254:54-61. 4. Maganty A, Byrnes ME, Hamm M, et al. Barriers to rural health care from the provider perspective. Rural Remote Health. 2023;23(2):7769. 5. Perez MV, Mahaffey KW, Hedlin H, et al; Apple Heart Study Investigators. Large-Scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909-17. 6. Wolf RM, Channa R, Liu TYA, et al. Autonomous artificial intelligence increases screening and follow-up for diabetic retinopathy in youth: the ACCESS randomized control trial. Nat Commun. 2024;15(1):421. 7. Scott AT, Kanaster K, Kaizer AM, et al. The utility of iCare HOME tonometry for detection of therapy-related intraocular pressure changes in glaucoma and ocular hypertension. Ophthalmol Glaucoma. 2022;5(1):85-93. 8. Levin AM, McGlumphy EJ, Chaya CJ, et al. The utility of home tonometry for peri-interventional decision-making in glaucoma surgery: case series. Am J Ophthalmol Case Rep. 2022;28:101689. 9. Mudie LI, LaBarre S, Varadaraj V, et al.: The ICare HOME (TA022) Study: performance of an intraocular pressure measuring device for self-tonometry by glaucoma patients. Ophthalmology. 2016;123(8):1675-84. 10. Ogle JJ, Hoo WCS, Chua CH, Yip LWL. Accuracy and reliability of self-measured intraocular pressure in glaucoma patients using the iCare Home tonometer. J Glaucoma. 2021;30(12):1027-32. 11. LaMonica LC, Bhardwaj MK, Hawley NL, et al. Remote screening for optic nerve cupping using smartphone-based nonmydriatic fundus photography. J Glaucoma. 2021;30(1):58-60. 12. Berneshawi AR, Shue A, Chang RT. Glaucoma home self-testing using VR Visual fields and rebound tonometry versus in-clinic perimetry and Goldmann applanation tonometry: a pilot study. Transl Vis Sci Technol. 2024;13(8):7. 13. Korot E, Pontikos N, Drawnel FM, et al. Enablers and barriers to deployment of smartphone-based home vision monitoring in clinical practice settings. JAMA Ophthalmol. 2022;140(2):153-60. 14. Chen E, Mills M, Gallagher T, et al; The Macustat Study Group. Remote patient monitoring of central retinal function with Macustat: a multi-modal macular function scan. Digit Health. 2022;8:20552076221132105. 15. Chen E, Mills M, Gallagher T, et al; The Macustat Study Group. Remote vision testing of central retinal acuity and comparison with clinic-based Snellen acuity testing in patients followed for retinal conditions. Digit Health. 2023;9:20552076231180727. 16. AREDS2-HOME Study Research Group; Chew EY, Clemons TE, Bressler SB, et al. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the eye (HOME) study. Ophthalmology. 2014;121(2):535-44. 17. Ferris FL, Wilkinson CP, Bird A, et al.; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844-51. 18. AREDS2-HOME Study Research Group; Chew EY, Clemons TE, Bressler SB, et al. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014;121(2):535-44. 19. Mathai M, Reddy S, Elman MJ, et al.; ALOFT Study Group. Analysis of the long-term visual outcomes of ForeseeHome remote telemonitoring. the ALOFT study. Ophthalmol Retina. 2022;6(10):922-9. 20. Ho AC, Schechet SA, Mathai M, et al. The predictive value of false positive ForeseeHome alerts in the ALOFT study. Ophthalmol Retina. 2023;7(2):196-8. 21. Liu Y, Holekamp NM, Heier JS. Prospective, longitudinal study: daily self-imaging with home OCT for neovascular age-related macular degeneration. Ophthalmol Retina. 2022;6(7):575-85. 22. Holekamp NM, de Beus AM, Clark WL, Heier JS. Prospective trial of Home OCT guided management of treatment experienced nAMD patients. Retina. May 24, 2024. [Epub ahead of print]. 23. Blinder KJ, Calhoun C, Maguire MG, et al. Home OCT imaging for newly diagnosed neovascular age-related macular degeneration: a feasibility study. Ophthalmol Retina. 2024;8(4):376-87. |

