 |
Q:
I have a 32-year-old patient with type 1 diabetes who presents for her first eye exam in many years. She is 12 weeks pregnant. What is the latest thinking on safely dilating and prescribing therapeutics if needed?
A:
During pregnancy, a woman may undergo development of new ocular conditions or modifications of existing ones. One that’s most commonly altered during pregnancy is diabetic retinopathy (DR). It has been well documented that patients with pre-existing diabetes, especially type 1, have an increased risk of the development or progression of DR during pregnancy.1,2 Rates of progression of DR may double during pregnancy, especially if retinopathy was present at conception.1
Given this transient increased risk of development or progression of DR during pregnancy and the first year post-partum, Caroline B. Pate, OD, professor and director of residency programs at the University of Alabama at Birmingham School of Optometry, advises to carefully monitor these patients by increasing the frequency of dilated exams.
The Clinical Practice Guidelines of the American Optometric Association recommend that women with diabetes who become pregnant should have a comprehensive eye and vision exam during every trimester with follow-up at six to 12 months postpartum.1,3,4 Due to the relatively short-lived nature of gestational diabetes, these patients do not carry the same risks of developing retinopathy during pregnancy and do not need to be monitored as frequently during pregnancy as those with pre-existing diabetes.
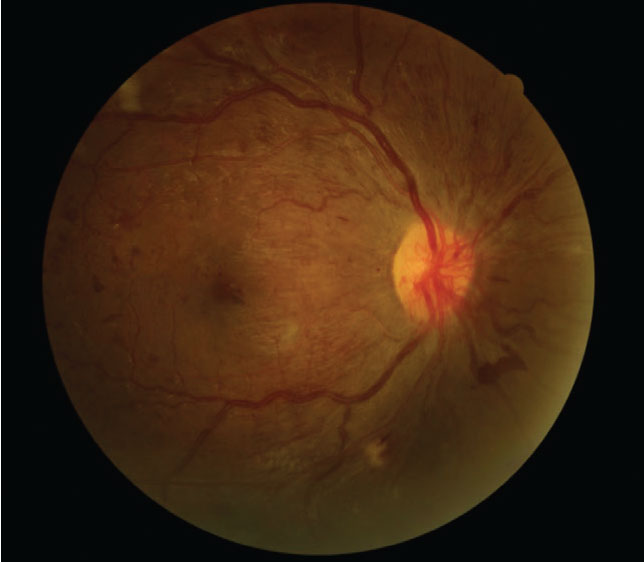 |
|
Despite 20/20 best-corrected vision, this type 1 diabetes patient who was seven months pregnant presented with proliferative DR. Click image to enlarge. |
Meds During Pregnancy
Although the historical risk of complications to the fetus as a result of using topical ocular diagnostic and therapeutic medications during pregnancy is low, one must still consider the risks and benefits prior to their use in this patient population.6
In June 2015, the FDA updated the prescription drug labeling for all new drugs from the “ABCDX” category designation in package inserts.7 Instead, the prescriber is now responsible for reading the package insert and analyzing the safety data to make an informed decision on the risks and clinical considerations when selecting which medication to use for a patient.
If ever in doubt, Dr. Pate advises, consult the patient’s ob-gyn/primary care physician before initiating treatment on a pregnant or nursing patient.
Due to the risks described above to a pregnant patient with pre-existing diabetes, dilation would certainly be warranted for your patient, despite diagnostic dilating agents traditionally holding the category “C” designation, prescribed only when the benefit justified the potential risk to the patient. Mydriacyl (tropicamide, USP) is available in 0.5% and 1% concentrations and could be used to dilate this patient. Avoid longer duration parasympatholytics such as homatropine and atropine due to the increased half-life.6 If able to dilate with tropicamide alone, avoid phenylephrine due to rare cardiovascular side effects, which have been reported especially with the 10% concentration.6 Keep in mind: punctal occlusion can help minimize systemic absorption of topical eye drops but do not prevent it completely. “Bottom line, don’t be afraid to dilate your pregnant patients,” Dr. Pate says.
Counsel all female diabetes patients of childbearing age about the associated risks of pregnancy on the progression or development of DR and the need for frequent monitoring during pregnancy, Dr. Pate says. If severe nonproliferative DR, proliferative DR or diabetic macular edema is detected, refer the patient to a retina specialist for treatment.
Dr. Ajamian is the center director of Omni Eye Services of Atlanta. He currently serves as general chairman of the education committee for SECO International. He has no financial interests to disclose.1. Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy. The Diabetes in Early Pregnancy Study Group. National Institute of Child Health and Human Development Diabetes in Early Pregnancy Study. Diabetes Care. 1995;18(5):631-7. 2. Sheth BP. Does pregnancy accelerate the rate of progression of diabetic retinopathy?: an update. Curr Diab Rep. 2008; 8:270-3. 3. AOA. Evidence-based Clinical Practice Guideline: Eye Care of the Patient with Diabetes Mellitus. www.aoa.org/AOA/Documents/Practice%20Management/Clinical%20Guidelines/EBO%20Guidelines/Eye%20Care%20of%20the%20Patient%20with%20Diabetes%20Mellitus%2C%20Second%20Edition.pdf. October 4, 2019. Accessed August 27, 2021. 4. Diabetes Control and Complications Trial Research Group. Effect of pregnancy on microvascular complications in the Diabetes Control and Complications Trial. Diabetes Care. 2000;23(8):1084-91. 5. Morrison JL, Hodgson LA, Lim LL, Al-Qureshi S. Diabetic retinopathy in pregnancy: a review. Clin Exp Ophthalmol. 2016;44(4):321-34. 6. Autry J. Pregnancy precautions: how to prescribe safely for new and expectant mothers. Review of Optometry. 2016;153(1): 28-31. 7. FDA Pregnancy and Lactation Labeling (Drugs) Final Rule. www.fda.gov/drugs/labeling-information-drug-products/pregnancy-and-lactation-labeling-drugs-final-rule. Accessed August 27, 2021. |

