Taking the Lead on GlaucomaJuly's issue is our 28th annual glaucoma report, where we explore the latest in treatments, technology, MIGS and diagnostic techniques to manage your patients with this aggressive disease. |
As medical optometry expands in scope, the role of the optometrist in glaucoma care is becoming more comprehensive. It is important for clinicians to be familiar with the diagnostic challenges associated with the disease and the tools that can better help manage these patients. In addition to making the correct diagnosis, ODs must be confident in our ability to effectively treat and comanage patients with various stages of glaucoma, especially end-stage.
Stages of Disease
To understand how to manage glaucoma, it is helpful to define the different stages of the disease. The following ICD-10 definitions are widely used and straightforward for clinicians to adapt in practice:1
Mild or early. Optic nerve abnormalities consistent with glaucoma
• but no glaucomatous visual field (VF) abnormalities on any VF test
• or abnormalities present only on short wavelength automated perimetry or frequency doubling perimetry.
Moderate. Optic nerve abnormalities consistent with glaucoma
• and glaucomatous VF abnormalities in one hemifield not within 5° of fixation (note: 5° = involvement of spots nearest fixation).
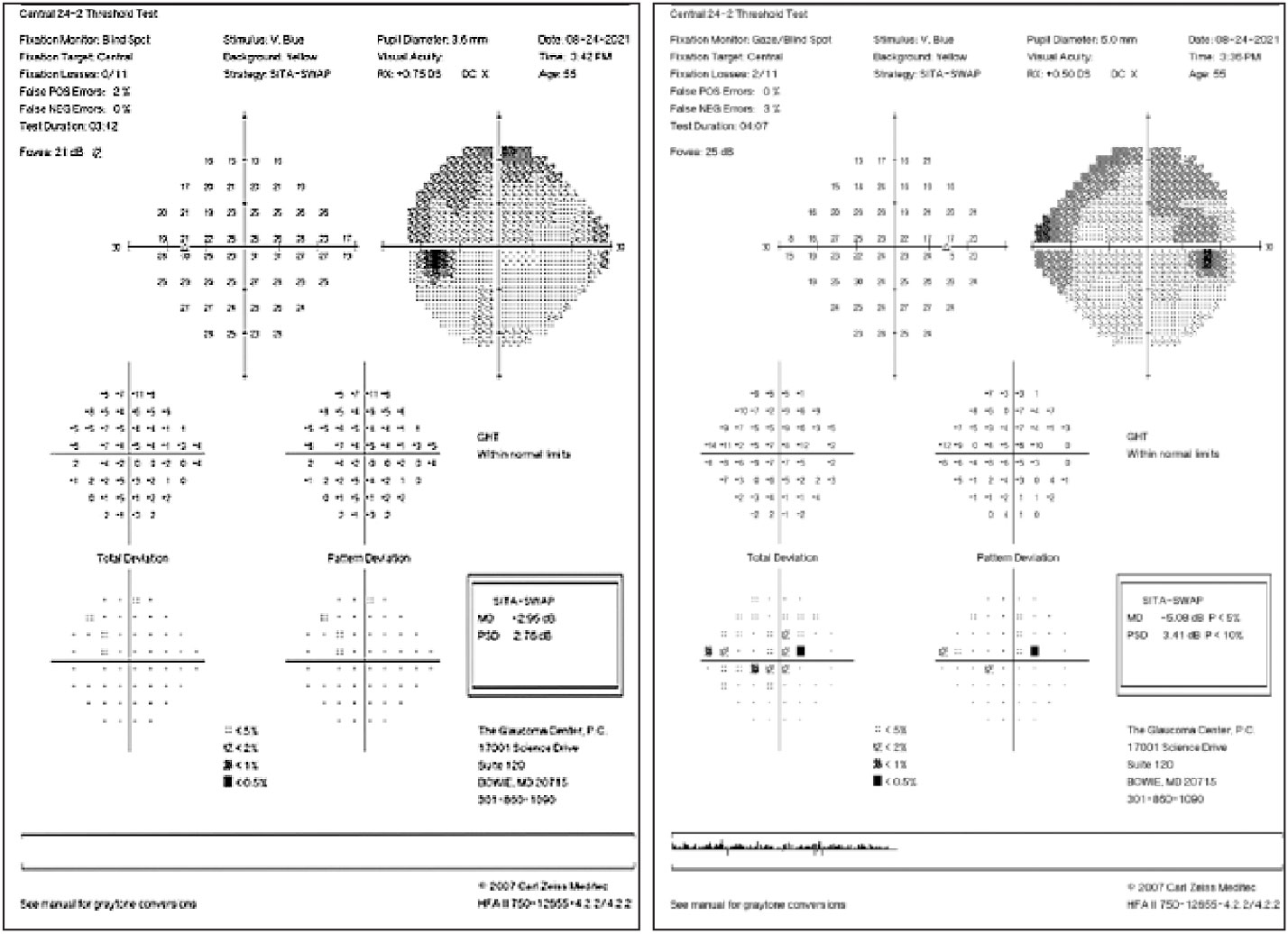 |
| SITA SWAP testing helps display early glaucomatous defects. Click image to enlarge. |
Advanced, late, severe. Optic nerve abnormalities consistent with glaucoma
• and glaucomatous VF abnormalities in both hemifields
• and/or loss within 5° of fixation in at least one hemifield.
At the initial glaucoma evaluation, disease severity must be clearly and correctly staged. Based on the clinical exam, diagnostic analysis and associated risk factors (e.g., age, race, central corneal thickness, family history, ocular comorbidities), an appropriate target pressure can then be determined accordingly.
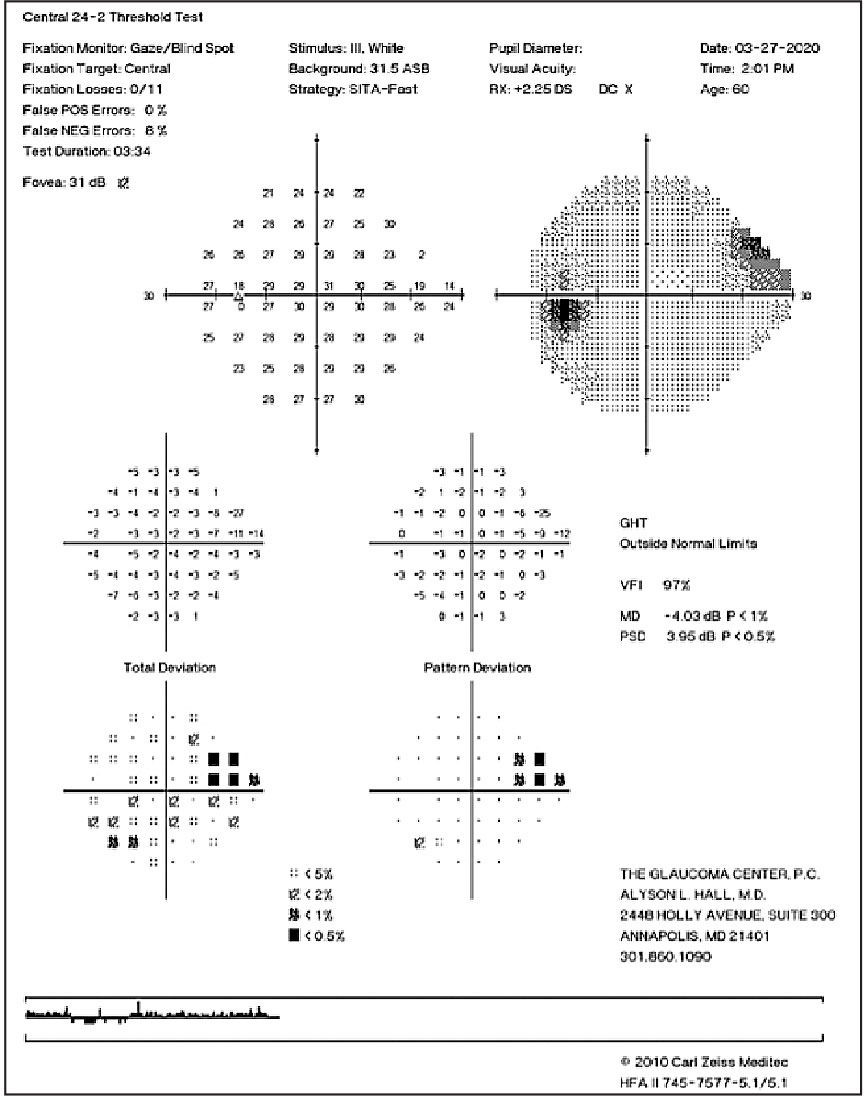 |
| Superior nasal step in a patient with moderate glaucoma. Click image to enlarge. |
Diagnostic OCT Challenges
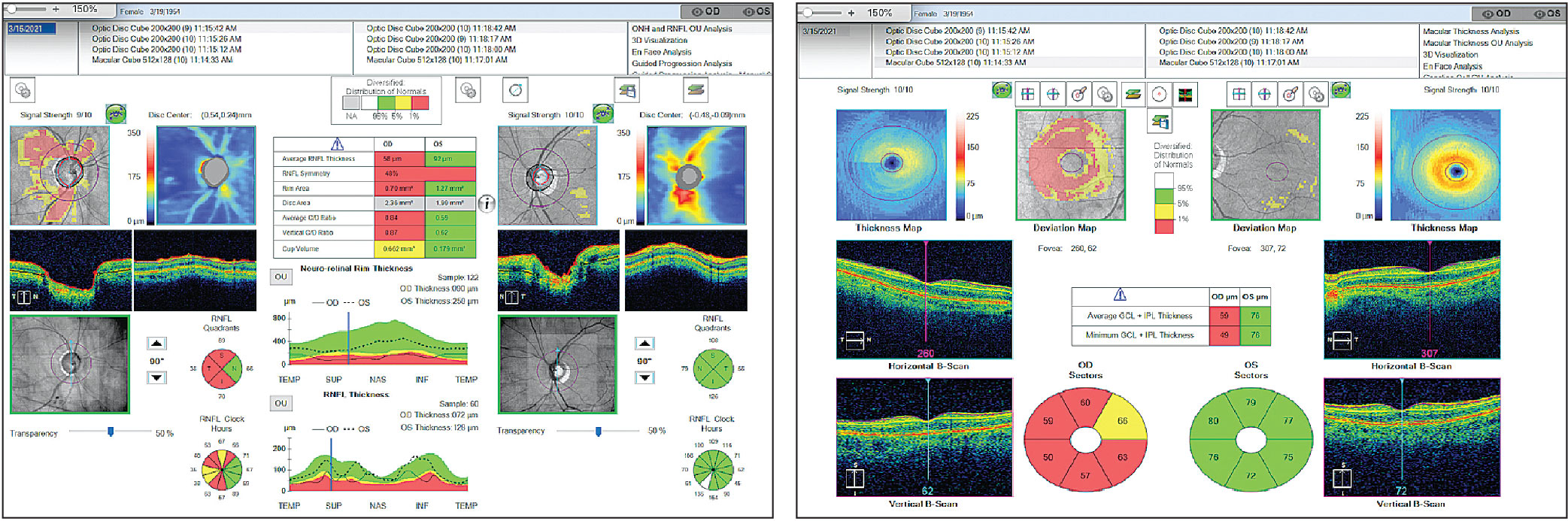
In early glaucoma, particularly pre-perimetric glaucoma, OCT of the retinal nerve fiber layer (RNFL) is extremely useful in showing early tissue loss. In advanced glaucoma, the OCT RNFL scan becomes less useful due to what is known as the “floor effect,” which means that loss of the nerve fiber layer may still occur but is no longer detectable on OCT. This is theorized to be caused by residual tissue, such as glial cells or blood vessels, being measured instead.2 The RNFL scan will not show progressive thinning greater than 40µm to 45µm, beyond which macular OCT ganglion cell analysis should be considered.3
Since central vision is typically intact until very late-stage disease, macular OCT can be helpful in tracking progression in severe glaucoma. Studies have shown that even with advanced structural damage and in eyes that have reached the floor effect with RNFL measurements, progression can still be detected with the macular OCT ganglion cell analysis algorithm.2,4 This is especially useful in patients who are poor VF test-takers or who are unable to complete VF testing due to physical or mental limitations.
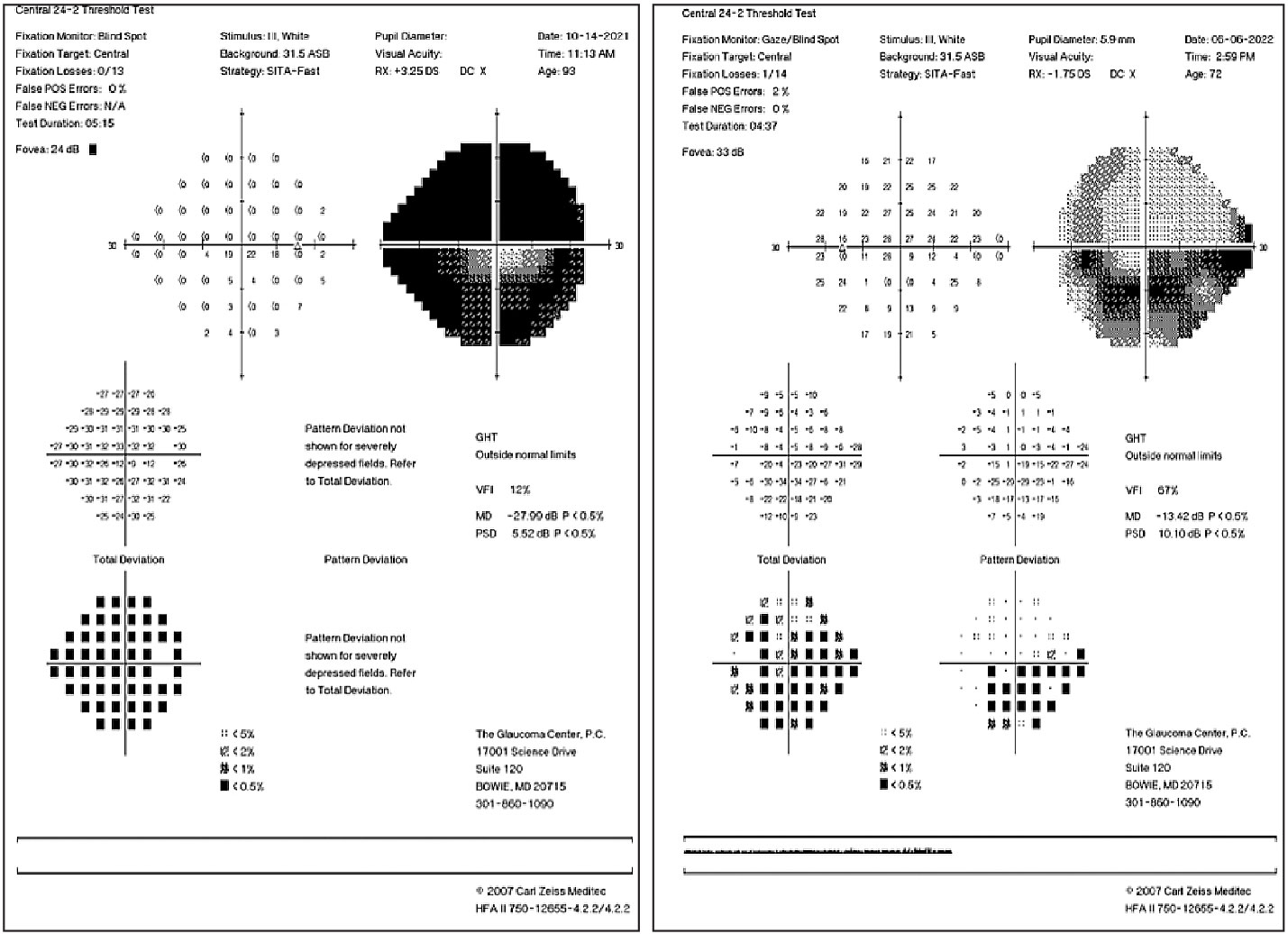 |
| Dense superior and inferior arcuate defects in an advanced glaucoma patient (left). Inferior arcuate defect involving fixation and early superior nasal step (right). Click image to enlarge. |
Diagnostic VF Challenges
In severe to end-stage glaucoma, the standard 24-2 VF is often completely depressed or with only a small central island remaining. In these cases, it is critical that the clinician switches to 10-2 VF testing to allow for more precise monitoring of the central island. The 24-2 field tests a total of 54 points that are 6° apart vs. the 68 points the 10-2 field is able to test 2° apart. Of the 54 points in the 24-2 algorithm, only 12 are tested within the central 10° of fixation. This is insufficient to allow for adequate monitoring of the central points in advanced disease, which help determine a patient’s ability to complete daily activities such as driving and reading.5 An effective alternative is the Humphrey 24-2C VF test, which includes the 24-2 grid but also incorporates 10 additional test points in the central 10° of vision.6
In early to moderate glaucoma, the rate of mean deviation (MD) change is comparable between the 24-2 and 10-2 fields. However, in eyes with severe disease, the rate of MD change is significantly higher on the 10-2 compared with the 24-2. The MD value is calculated from a subset of the total points. In the 24-2, the points are mostly central and midperipheral, whereas in the 10-2, the points are all within the central 10°. Therefore, the central VF loss in more severe disease is not able to be followed for progression with the 24-2 test.5
In advanced disease, it is necessary to take VF test consideration one step further. When VF loss is very severe, the standard size III automated perimetry may no longer be adequate to catch progression. Switching to a Fastpac size V stimulus with 10-2 testing allows for a larger target size so that points that previously did not respond to a size III target will now show a response. This is typically reserved for end-stage glaucoma when there is only a small central island remaining.3
While there is a need for at least three tests the first year of glaucoma diagnosis to pinpoint progression, much of the frequency of testing in advanced disease depends on the stability of the patient. If a severe glaucoma patient has been stable for many years with well-controlled intraocular pressure (IOP), VF testing twice a year may be sufficient. Contrast that with a severe glaucoma patient whose IOP is above ideal, or whose VF shows deterioration, more follow-ups are required to monitor for changes in IOP fluctuation, and more frequent fields are required to confirm progression.
 |
| Left: Severe glaucomatous optic nerve damage of the right eye with a very thin RNFL, which may not show further thinning past this point. Right: Macula ganglion cell complex, which can help provide information about progression in the same individual. Click image to enlarge. |
Medical Management
Setting target pressures can sometimes be challenging in advanced disease; however, we can reference major landmark studies such as the Advanced Glaucoma Intervention Study for guidance. While there is no magic number for the perfect target IOP in advanced disease, we know from the study that patients who consistently maintained an IOP of less than 18mm Hg at every visit over six years, with a mean IOP of 12.3mm Hg, had good VF preservation.7 As a general rule of thumb, a target IOP in the low teens is a good initial goal for severe disease. Other factors, of course, contribute to this goal. For example, a young monocular patient with a thin central corneal thickness and strong family history of glaucoma may require a single-digit target IOP. Compare this case to an elderly patient in poor systemic health with a thick central corneal thickness and other ocular comorbidities who may not necessarily require aggressive therapy to lower IOP to the low teens.
It is important to change this target pressure accordingly depending on how the patient progresses. An IOP in the low teens may be appropriate at first for a severe glaucoma patient, but if their VF shows consistent progressive defects, a more aggressive treatment goal needs to be implemented.
In addition to setting appropriate treatment goals for each patient, it is also important to comanage any surgical candidates with ophthalmology, a process that should begin as soon as possible to avoid consequential delays. It is beneficial for optometrists treating glaucoma to establish a good relationship and frequent communication with a glaucoma surgeon to encourage more timely and effective intervention as needed. In cases where IOP is inadequately controlled on maximum-tolerated medical therapy and still elevated after laser intervention, prompt referral is necessary for consideration of incisional glaucoma surgery such as a trabeculectomy or tube shunt surgery.
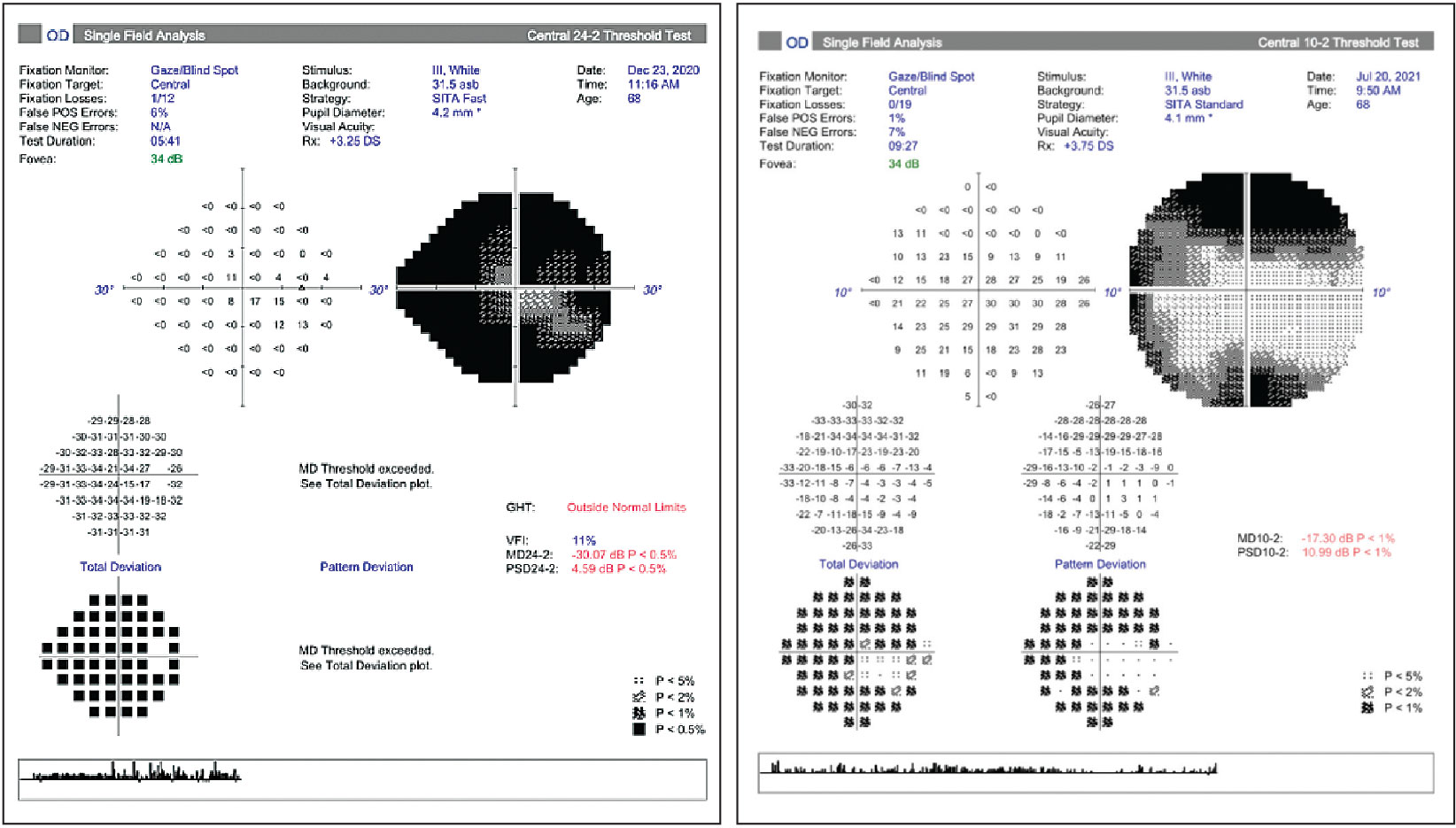 |
| Left: Small central island in advanced glaucoma. Right: The same patient was assessed with 10-2 testing to allow for more points in the remaining central island to be monitored. Click image to enlarge. |
Topical Therapy Considerations
Clinicians must acknowledge the critical role that the patient plays in their own glaucoma treatment success. In early- to moderate-stage glaucoma, it is easier for patients to keep track of their eye medication(s) and be compliant with their therapy, which typically only involves one or two drops. In severe glaucoma, it is more common for patients to be on multiple drops. As we know, compliance becomes more difficult with more complex eye drop regimens.8 In these situations, it is wise to consider fixed-dose combination agents to increase adherence. Switching to combination drop therapy when the patient requires more than a single agent to lower IOP will significantly simplify the regimen and decrease the number of trips to the pharmacy to increase the likelihood of better outcomes overall.
It is also important to consider the ocular surface when treating glaucoma. The majority of glaucoma drops contain benzalkonium chloride, which can be damaging to the ocular surface and lead to or exacerbate existing ocular surface disease (OSD).9 This outcome is of significant concern in the elderly population, which is already at risk for dry eye disease.9 Approximately 40% to 59% of glaucoma patients on topical medications have OSD, which can significantly impact their quality of life.10 Using a preservative-free formulation can help decrease ocular surface toxicity and irritation and improve patient satisfaction with the recommended therapy.11 By encouraging patient compliance with the prescribed regimen, the likelihood of incisional surgery decreases. As glaucoma surgery carries risks of failure, infection and vision loss, the importance of a well-tolerated topical regimen cannot be understated.
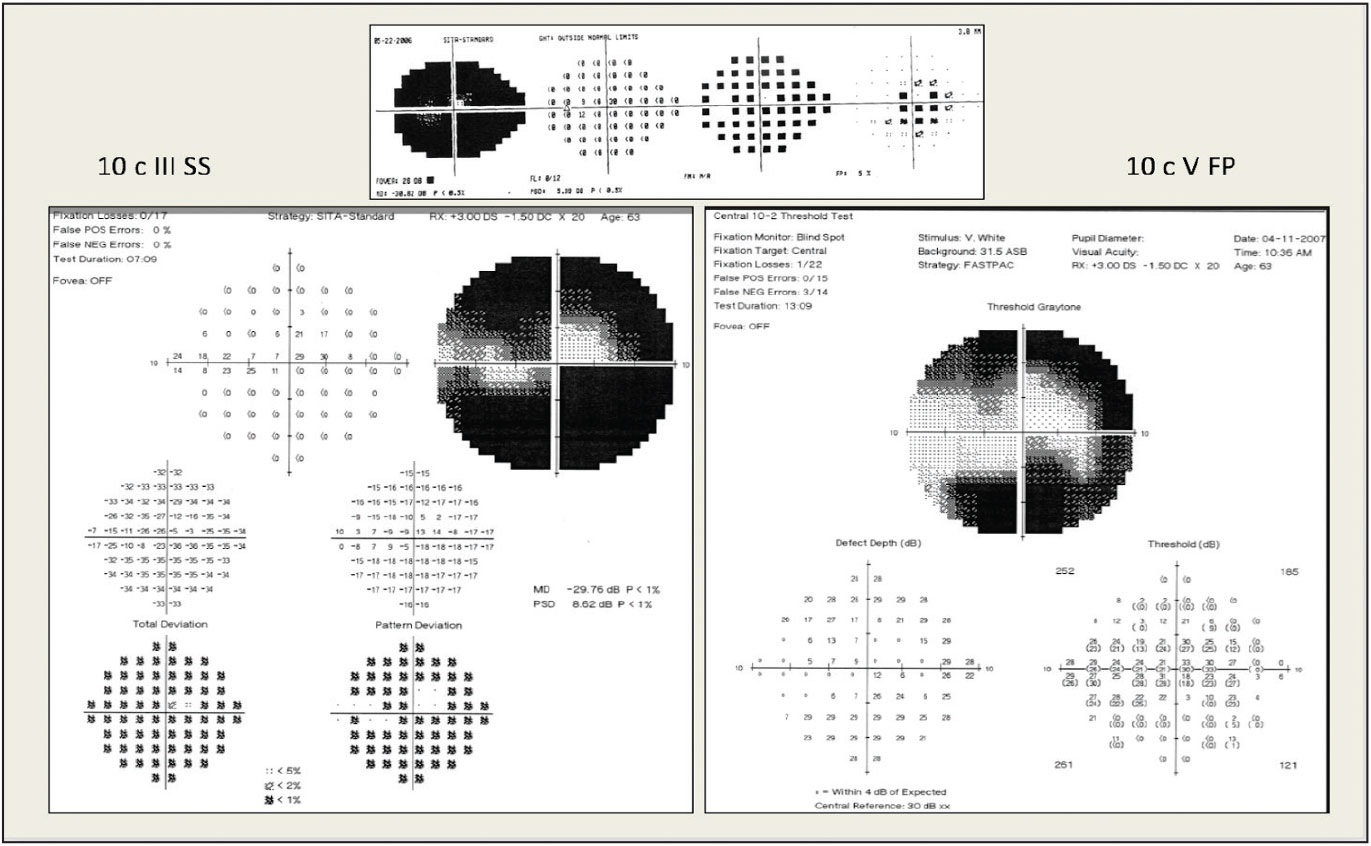 |
| Pictured here is 24-2 testing (top) and 10-2 testing (bottom left). Fastpac size V stimulus testing allows for better monitoring of the central remaining points (bottom right). Photo: Peter Lalle, OD. Click image to enlarge. |
Takeaways
Glaucoma is the second leading cause of blindness worldwide, and about three million Americans currently live with it, a number that will grow as the population ages and lives longer.10 This is a disease that optometrists encounter regularly and may find rewarding to manage as it establishes a lifelong relationship between the doctor and the patient, whose quality of life may improve with the appropriate treatment regimen.
It is important that optometrists who manage glaucoma understand the full spectrum of the disease and know how to best implement diagnostic tools to supplement clinical examination, especially in advanced cases when time is even more of the essence. Understanding when it’s necessary to refer for laser or surgical intervention is equally as important to treatment success.
Dr. Hu graduated from the University of California Berkeley School of Optometry and completed her residency in ocular disease at the Baltimore Veterans Affairs Medical Center. She currently practices at the Glaucoma Center in Bowie, MD, and is a speaker for Aerie Pharmaceuticals.
1. Glaucoma quick reference guide. American Academy of Ophthalmology. www.aao.org/assets/706a6588-8001-4155-a24b-3eddad58c0f7/635672098930070000/glaucoma-icd-10-quick-reference-guide-pdf. Accessed June 3, 2022. 2. Bowd C, Zangwill LM, Weinreb RN, et al. Estimating optical coherence tomography structural measurement floors to improve detection of progression in advanced glaucoma. Am J Ophthalmol. 2017;175:37-44. 3. Francis BA, Chopra V. Glaucoma progression detection, part 2. Ophthalmology Management. November 1, 2018. [Epub ahead of print]. 4. Lavinsky F, Wu M, Schuman JS, et al. Can macula and optic nerve head parameters detect glaucoma progression in eyes with advanced circumpapillary retinal nerve fiber layer damage? Ophthalmology. 2018;125(12):1907-12. 5. Rao HL, Begum VU, Khadka D, et al. Comparing glaucoma progression on 24-2 and 10-2 visual field examinations. PloS One. 2015;10(5):e0127233. 6. Phu J, Kalloniatis M. Ability of 24-2C and 24-2 grids to identify central visual field defects and structure-function concordance in glaucoma and suspects. Am J Ophthalmol. 2020;219:317-31. 7. The Agis Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429-40. 8. Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122(7):1308-16. 9. Bonniard AA, Yeung JY, Chan CC, Birt CM. Ocular surface toxicity from glaucoma topical medications and associated preservatives such as benzalkonium chloride (BAK). Expert Opin Drug Metab Toxicol. 2016;12(11):1279-89. 10. Zhang X, Vadoothker S, Munir WM, Saeedi O. Ocular surface disease and glaucoma medications: a clinical approach. Eye Contact Lens. 2019;45(1):11-18. 11. Thygesen J. Glaucoma therapy: preservative-free for all? Clinical Ophthalmol. 2018;12:707-17. |

