The uvea, a highly vascularized section of the eye located beneath the sclera, supplies most of the ocular structures with nutrients via the anterior and posterior branches of the ophthalmic artery.
The uvea consists of the iris, ciliary body and choroid. The iris controls how much light enters the eye, while the ciliary body produces aqueous humor and controls its outflow by contracting and widening the trabecular meshwork. The ciliary body also controls accommodation by contracting and relaxing.
The third element, the choroid, is a highly vascularized and pigmented tissue that provides nourishment to the outer retinal layers and absorbs excess light. Inflammation of any of these structures is known as uveitis.
One synonym of uveitis is iritis, and although iritis is more technically and anatomically specific, clinicians often use the terms interchangeably. The most common form of this disease is nongranulomatous anterior uveitis, which can present as unilateral or bilateral; chronic or acute; and idiopathic, infectious, immunological or neoplastic.
Classic symptoms include redness, photophobia and pain often described as dull ache; however, with chronic forms of the disease, these symptoms may be completely absent. Often, a sufficient medical and ocular history can reveal the precipitating cause, although even laboratory testing cannot uncover the underlying etiology in every instance.
Regardless, it is important to properly classify the uveitis in order to correctly diagnose and treat the patient, thus eliminating the potential for further complications, including blindness. This article will review typical signs and symptoms of anterior uveitis, as well as discuss essential treatment considerations.
| |
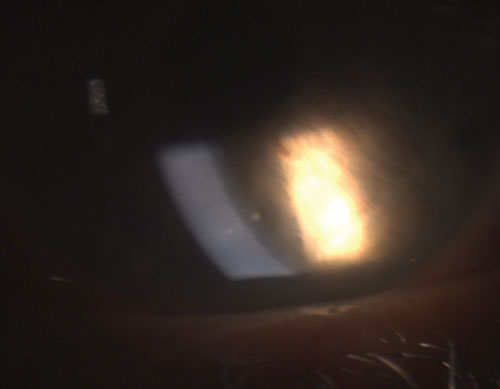
| 1. Small, resolving mutton-fat keratic precipitate (KP) in granulomatous uveitis. | |
| |
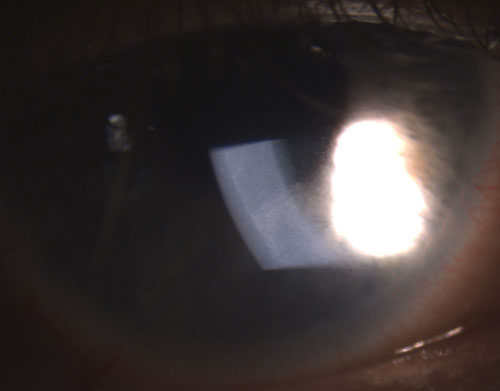
| 2. Fine KPs on the endothelium in non-granulomatous uveitis. | |
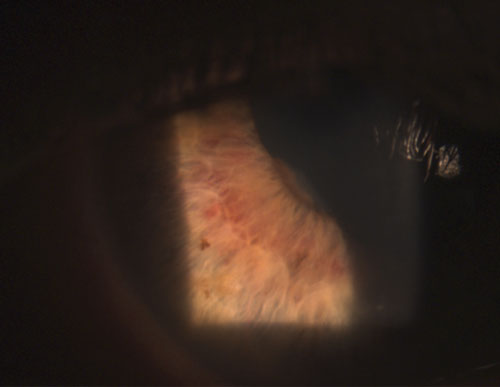 | |
| 3. Area of posterior synechia (iris adhered to lens), with dilated stromal iris vasculature. | |
 | |
|
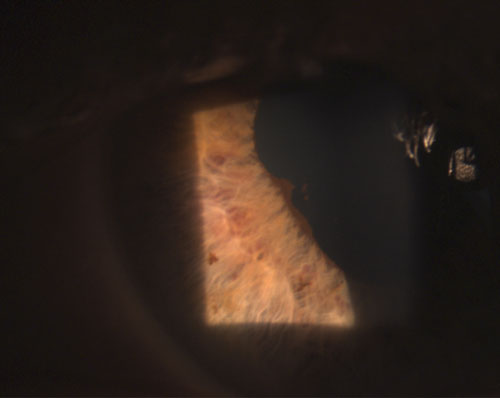
4. Posterior synechia partly broken after instillation of phenylephrine 10% and atropine 1%. |
Disease Classification, Workup and Diagnosis
When diagnosing anterior uveitis, you must consider a variety of presenting signs and associated features. As previously noted, characteristic symptoms include pain in the form of a dull ache, redness and photophobia. Visually, the customary ciliary flush (circumlimbal flush) is often seen and the pupil may be mid-dilated. For an official diagnosis, however, cells must be seen in the anterior chamber, and flare may or may not be present. It is important to note that flare sometimes can be seen in the anterior chamber when no active inflammation is present, because long-standing, chronic uveitis damages the vasculature integrity of the iris and ciliary body.1
To properly diagnose and manage uveitis, you must first categorize it. Anterior inflammation confined to the iris and anterior chamber is termed iritis. When the inflammation also involves the ciliary body, as evidenced by the presence of anterior vitreous cells, it is called iridocyclitis. However, when only the ciliary body is inflamed, it is simply called cyclitis (although this is not usually a clinically significant term). Intermediate uveitis, or pars planitis, involves inflammation of the pars plana, the middle portion of the ciliary body. Aggregates of white blood cells, or snowball opacities, accumulated near the inferior retina are typically seen in pars planitis.1
Posterior uveitis involves inflammation in the posterior segment, including the retina, choroid, vitreous and sometimes sclera, while pan-uveitis involves all structures of the uvea in addition to adjacent tissues. It should be noted the further posterior the uveitis proceeds in the eye, the greater the risk of associated systemic disease, the more difficult it will be to treat, and the greater the risk of complications. In any case, the difference between the specific semantics helps the doctor with clinical diagnosis and directs attention to the appropriate areas of concern.
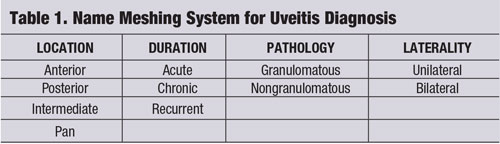
A “name meshing” strategy (see “Name Meshing System for Uveitis Diagnosis.") is one tool that can be used to focus clinical thought and provide a tailored, cost-effective evaluation and management of the patient’s disease.2-4 The uveitic entity should be broken down by its location, duration, pathology and laterality.1-4
In reference to ocular inflammation, “granulomatous” typically refers to a more severe form of uveitis, with distinctive features like iris granulomas, Koeppe nodules on the pupillary margin, Busacca nodules in the iris stroma and keratic precipitates (KPs) on the corneal endothelium that are large, globular and greasy, known as mutton-fat KPs (figure 1).1-3,5 A hypopyon may form in the anterior chamber if the inflammation goes unchecked.
Common granulomatous uveitides include tuberculosis, sarcoidosis and Lyme disease.2 Nongranulomatous ocular inflammation, on the other hand, is less severe and is characterized by smaller KPs (figure 2), fewer (if any) nodules and decreased probability of synechiae formation (figures 3 and 4).2,3 Overall, granulomatous uveitis is more likely to be associated with systemic disease and more difficult to treat with greater risk for complications than nongranulomatous uveitis.
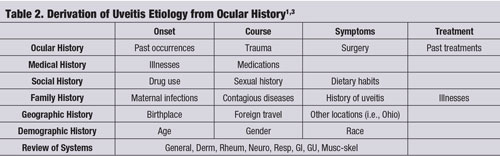
Frequently, a specific ocular history—in conjunction with a careful slit lamp examination—can lead to the correct etiology of the uveitis without any further testing or laboratory studies. (See “Derivation of Uveitis Etiology from Ocular History,” page 60.)1
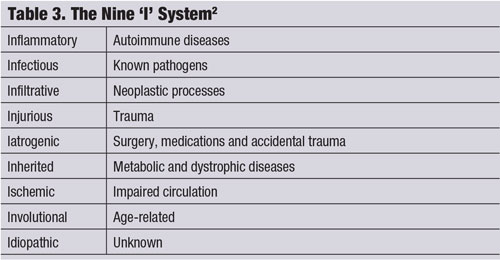
The “Nine I system,” which categorizes inflammation of ocular tissues into a specific heading, is another useful classification instrument (see “The Nine ‘I’ System.") Any of the nine “I’s” can be the root cause of uveitis, with up to 38% of the cases being idiopathic.6
Bloody Cues
Acute nongranulomatous uveitis can be associated with the human leukocyte antigen B27 (HLA-B27). Additional entities that can be linked to HLA-B27 include Reiter syndrome, inflammatory bowl disease (i.e., ulcerative colitis or Crohn’s disease), ankylosing spondylitis, Behçet disease and psoriatic arthritis.1,6,7
These entities are usually anterior and unilateral. Other acute nongranulomatous uveitides include Lyme disease and trauma. Common chronic nongranulomatous entities include juvenile rheumatoid arthritis and Fuchs’ heterochromic iridocyclitis.3,4,6
Chronic uveitis is usually less symptomatic than acute presentations. Although syphilis is typically lumped into chronic granulomatous uveitis along with sarcoidosis and tuberculosis, it can present in any of the categories as a masquerader.4,6
Posterior uveitides include tuberculosis, toxoplasmosis, histoplasmosis, sarcoidosis and herpes––although herpes is often anterior as well.3,6 Although usually posterior, any form of posterior uveitis can rarely present anteriorly. An entity that may be miscategorized as idiopathic anterior uveitis is glaucomatocyclitic crisis (i.e., Posner-Schlossman syndrome).1 This is classically a white eye with a mild anterior chamber reaction and highly elevated IOP (30mm Hg to 60mm Hg). It tends to be unilateral, recurrent and relatively asymptomatic.1,5
Blood studies often must be ordered in cases where the history and physical examination do not lead to a definitive diagnosis––especially in the presence of bilateral, granulomatous or recurrent uveitis.6 The “shotgun” approach is costly, nonspecific and can easily be avoided with astute clinical skills.
|
|
|
|
|
|
Here are some of the most common laboratory tests used to help localize the etiology of uveitis:6-8
• Complete blood count (CBC) with differential helps to determine the patient’s general health status, and can aid in diagnosing a variety of disorders, such as anemia, infection and leukemia.
• C-reactive protein (CRP) is a marker for inflammation and serves as a treatment response monitor.2,4 CPR tests are unable to determine the cause or location of the inflammation within the body, however.
• Erythrocyte sedimentation rate (ESR) is typically ordered in conjunction with a CRP test. An ESR helps detect inflammation and serves as a monitor for the underlying etiology.
• Antinuclear antibody (ANA) testing screens for certain autoimmune disorders like systemic lupus erythematosus, scleroderma, juvenile arthritis, polymyosistis, inflammatory bowel disease and psoriasis.
• Rheumatoid factor (RF) testing can help you diagnose rheumatoid arthritis and Sjögren’s syndrome, among others that sometimes overlap with ANA testing.
• Rapid plasma regain (RPR), venereal disease research laboratory (VDRL) and fluorescent treponemal antibody absorption test (FTA-ABS) are used to screen for syphilis. The FTA-ABS is a 24-hour test used to detect antibodies to the bacteria Treponema pallidum and confirm the presence of syphilis. FTA-ABS does not indicate if the disease is active or inactive, however, and is typically administered after a screening test for active disease, RPR or VDRL. Other treponemal tests are available, such as microhemagglutination-Treponema pallidum (MHA-TP), Treponema pallidum particle agglutination assay (TP-PA) and Treponema pallidum hemaglutination assay (TPHA), but are not as commonly known in the eye care communities as FTA-ABS.
|
Both RPR and VDRL typically register positive in primary and secondary syphilis and negative in tertiary (latent) syphilis and after successful treatment of the disease.4 RPR is commonly chosen over VDRL because it is both easier to administer and less expensive. False positives can occur, however, with both tests. Entities that may disrupt the accuracy of the results include but are not limited to tuberculosis, malaria, lymphoma, lupus, Lyme disease, viral infections, connective tissue diseases, IV drug use and pregnancy.
• Angiotensin-converting enzyme (ACE) supports a sarcoidosis diagnosis and helps to monitor disease activity during treatment. A serum lysozyme test can also be used to test for sarcoidosis.
• Chest X-ray (CXR) or computed tomography (CT) scan is also helpful when investigating for tuberculosis or sarcoid nodules in the lungs.6 Normally, when I order a CXR to rule out sarcoid, I will request posterior-anterior and lateral (PA/LAT) images. In any case, it is important to annotate what you are looking for (i.e., rule out sarcoid nodules in setting of bilateral uveitis) to help direct the radiologist in his or her examination. In a hospital setting, it is also paramount when ordering these tests to engage the patient’s primary care physician (PCP) or appropriate specialist if abnormalities are found.
For eye doctors who do not have access to labs or imaging, sending the patient to his or her PCP with exact recommendations of what tests to order and why will aid in expediency for the patient and help guide the PCP who relies on your expertise for ophthalmic conditions.
• Human leukocyte antigen B27 (HLA-B27) is found on the surface of white blood cells and is associated with a number of autoimmune disorders, such as ankylosing spondylitis and Reiter syndrome. It is not necessarily a specific marker for any one disease, however, and can actually be the singular determining factor in some iritis cases.4
• Purified protein derivative (PPD) tests for latent tuberculosis.
• Lyme titre and enzyme-linked immunosorbent assay (ELISA), together with anti-Borrelia burgdorferi immunoglobulins M and G (IgM/IgG), are used to detect the presence of Lyme disease. ELISA also can be used to detect HIV, as can the Western Blot test.2,7
Other uncommon tests for uveitis that may have relevance in certain patient presentations include a Sjögren’s antibody (SS-A, SS-B) profile, urinalysis and a search for viral entities like cytomegalovirus (CMV) IgG/IgM antibodies. Recall from immunology that IgG represents past exposure or immunity to a disease, and IgM represents recent exposure or likely active infection.
Management of Non-Traumatic Uveitis
First episodes of mild, unilateral iritis are often idiopathic and associated with a viral or sinus infection, or traumatic event. Further diagnostic testing usually can be curbed, because observation and treatment of symptoms is typically sufficient in these low-risk cases.2
Depending on the severity of inflammation with a traumatic iritis case, anti-inflammatory medicines sometimes can be withheld. However, sound clinical judgment must be exercised when determining if and when medication use is necessary.
For any therapeutic endeavor, a specific treatment goal is key. Obviously, increasing patient comfort is paramount in uveitis care. The fundamental purpose in uveitis management hinges on reducing inflammation, thus decreasing morbidity and the likelihood of other, more serious complications, such as vision loss and glaucoma.
In light of these goals, four main objectives should be considered when treating an iritis patient:
• Decrease pain.
• Prevent posterior synechiae and thus pupillary block.
• Prevent peripheral anterior synechiae (PAS) and thus angle closure.
• Re-establish the blood-aqueous barrier.
Atropine and other similar cycloplegics/mydriatics play an integral role in all four objectives.4 Cycloplegic agents act on the vasculature to help stabilize the blood-aqueous barrier, preventing further leakage. By immobilizing the iris, along with their action in ciliary muscle paresis, cycloplegics not only help with pain control, but their dilating effect is equally important in thwarting angle closure and pupillary block by averting iris to lens adhesion. Corticosteroids (usually topical for most cases of anterior uveitis) reduce the body’s inflammatory response and are a mainstay in iritis care. They also help reduce capillary permeability and vasodilation.4,7,13
Other therapeutic options include nonsteroidal anti-inflammatory drugs (NSAIDs), immunosuppressive/immunomodulatory agents and surgical options (e.g., laser peripheral iridotomy or periocular implant).2,3,9,12 Corticosteroid use in uveitis normally needs to be tapered in order to prevent rebound inflammation.
Early, frequent steroidal administration classically is prescribed to guarantee a suitable loading dose in order to aggressively quell the inflammation. Tapering appropriately, according to clinical response, ensures the proper remission of the uveitis without a rebound of inflammation.3 However, if a steroid is prescribed in traumatic uveitis, it is generally over a brief time period, eliminating the necessity of medication tapering––especially since the inflammatory stimulus (trauma) is gone.
Increased IOP and posterior subcapsular cataract (PSC) are two primary concerns associated with corticosteroid use; however, these complications are not routinely seen following short-term use. Likewise, increased IOP with concurrent corticosteroid use does not always mean that the steroid is the cause of the elevation. In uveitis, IOP generally is lower than normal––although in some cases, it can be higher than normal, depending on when in the disease process the patient presents for care.1,4,11 Two possible reasons for decreased IOP include:1,4
• An increase in the release of endogenous prostaglandins augments uveoscleral outflow.
• A decrease in aqueous humor production by the inflamed ciliary body.1,4
Potential explanations for increased IOP include:
• Clogging of the trabecular meshwork with inflammatory cells and protein.
• Trabeculitis, or inflamed, swollen meshwork fibers.
• Posterior synechiae.
• Peripheral anterior synechiae.
• Steroid-induced IOP elevation.
• The fact that the “sick” eye is returning to normal.
An IOP rise can occur during the corticosteroid treatment period, but it is not always secondary to the side effects of the corticosteroid itself. As such, the term “steroid-responder” is sometimes wrongly attributed to the healing eye’s normalization of aqueous production before the trabecular meshwork has totally phagocytized the white blood cells and fibrinous protein remnants from the drainage angle.4 Prematurely stopping the steroid treatment prior to complete inflammatory resolution may, in fact, do more harm than good; instead, the steroid treatment should be maintained and an IOP-lowering medication (i.e., aqueous suppressant), such as a beta-blocker or carbonic anhydrase inhibitor, should be added.
It is important to note prostaglandin analogs and miotics should be avoided in uveitis, because they may increase inflammation.8,10-12 Miotics also increase the risk of posterior synechiae formation.1 Adrenergic agonists, such as brimonidine and apraclonidine, generally are safe to use in uveitis patients with increased IOP.
When dealing with anterior uveitis, the exact dosing schedule is more art than science, as each case can present differently and slightly nuanced strategies can produce similar, positive results. One typical treatment protocol includes the administration of one drop of prednisolone acetate 1.0% every hour for two to three days, or until mild cells (< grade 2) are seen. Then, planned tapering of the steroid can be accomplished by continually cutting the administration frequency in half every third day.
For milder presentations, loteprednol etabonate gel 0.5% administrated at a less frequent dosing schedule might be best. For more severe or recalcitrant cases, however, difluprednate ophthalmic emulsion 0.05% QID or Q2H may be appropriate. Some patients may even require oral corticosteroids; a common choice is prednisone, which usually is dosed between 20mg to 40mg at a frequency of BID to QID for several days. When prescribing oral corticosteroids, take note of any systemic illnesses, as well as other medications that the patient is using, in case of side effects or interactions between the different medicines.
As such, you may want to consult with the patient’s PCP prior to prescribing oral corticosteroids. Consideration also should be given to prescribing an antihistamine that acts to inhibit stomach acid production, such as ranitidine (Zantac, GlaxoSmithKline), in order to prevent gastrointestinal upset. At the very least, ensure the patient takes the oral corticosteroid with some food or milk.
As previously mentioned, cycloplegics, such as homatropine 5.0% or atropine 1.0%, are necessary for proper uveitis management. A common approach may include one drop of homatropine 5% TID for three days, BID for two days and QD for one day; however, an extended period over several weeks may need to be employed for more severe cases. Remember, because the eye is inflamed, a dosing strategy that is greater than the half-life of the medication will be needed. This is because the medicine is being metabolized at a much faster rate in a sick eye. Depending on severity, the patient should return for a follow-up visit in two to five days initially, then as needed.
Management of Traumatic Uveitis
The frequency of uveitis in the United States matches international numbers at approximately 15 cases per 100,000 persons.6,7
Trauma is the third most common cause of anterior uveitis.6,7
Morbidity generally results from symptoms, posterior synechiae, cystoid macular edema, increased IOP with resultant glaucoma, cataract formation and retinopathy.4,6,9
Other complications associated with traumatic iritis include hyphema, iridodialysis, iridoschisis, lens dislocation and/or opacification, commotio retinae, optic neuropathy, posterior vitreous detachment, retinal tears and detachments, choroidal rupture, corneal edema and angle recession.5,9-11
Hyphema, if present, is a serious condition requiring close monitoring. Patients are typically confined to bed rest with limited activity, where their head should be elevated at least 30° and a shield placed over the eye for enhanced protection. Patients should avoid aspirin, but can take acetaminophen for pain as needed.
Atropine 1.0% should be in-stilled QD to TID and prednisolone acetate 1.0% dosed Q2H to QID. Oral aminocaproic acid, an antifibrinolytic, also should be administered, depending on the size of the hyphema.10-12
Laboratory studies also should be considered for hyphema cases. Typical labs ordered include complete blood count (CBC) with differential, prothrombin time (PT), partial thromboplastin time (PTT), blood urea nitrogen (BUN), creatinine, electrolytes, sickle prep and hemoglobin studies. IOP medications (i.e., beta-blockers) also should be instituted if IOP is elevated significantly. Prostaglandins and miotics should be avoided, because they can add to the inflammatory effect.8,10-12
Angle recession is noted if there is an uneven iris insertion posteriorly, allowing a larger-than-normal band of ciliary body to be seen.8 This can be confirmed if the contralateral eye’s gonioscopic findings are normal. Angle recession does not always occur with blunt trauma, and––if present––does not always produce an elevated IOP at onset. However, microscopic damage to the trabecular meshwork endothelial cells is possible, with resultant IOP increase years after the initial trauma.8,10 Regular eye examinations are needed to monitor these patients for future complications.
Uveitis may present concurrently with other morbidities. Therefore, it is prudent to be thorough when examining a patient with anterior segment inflammation. A methodical case history often can pinpoint the cause of the inflammation. Additionally, knowing what to look for during the slit lamp evaluation is imperative, so proper management and further diagnostic testing, if needed, can be instituted in an expedited manner.
Iritis can range from mild to severe, with vision loss and even blindness occurring if left untreated. Most cases of iritis that present to the primary eye doctor are localized anteriorly, mild to moderate in severity and relatively simple to manage. The prognosis generally is favorable with appropriate treatment and follow-up regimens; the pillars for proper management remain corticosteroids and cycloplegics. Bilateral and recurrent cases may need further investigation into the etiology. Overall, it is vital to educate patients about symptoms and the importance of future periodic eye examinations to monitor for complications.
Dr. Dohm is the department head of optometry and the associate director for medical services at Naval Hospital Oak Harbor on Naval Air Station Whidbey Island in Oak Harbor, Wash. He has no direct financial interests in any of the products mentioned.
1. Yanoff M, Duker JS (eds.). Ophthalmology, 2nd ed. St. Louis: Mosby; 2004.
2. Medscape. Uveitis Classification. Available at: http://emedicine.medscape.com/article/1208936-overview. Accessed November 8, 2012.
3. Smith RE, Nozik RM. Uveitis: A Clinical Approach to Diagnosis and Management. Baltimore: Williams & Wilkins; 1983.
4. Harkins TJ. Personal communication. Kansas City Veterans Administration Medical Center; Kansas City, MO. Aug-Dec 2005.
5. Gold DH. Clinical Eye Atlas. Chicago: AMA Press; 2002.
6. Medscape. Iritis and Uveitis. Available at: http://emedicine.medscape.com/article/798323-overview. Accessed November 10, 2012.
7. Medscape. Uveitis evaluation and treatment. Available at: http://emedicine.medscape.com/article/1209123-overview. Accessed November 10, 2012.
8. Kunimoto DY. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease, 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
9. Kanski JJ. Clinical Ophthalmology: A Systematic Approach. 5th ed. London: Butterworth Heinemann; 2003.
10. Shingleton BJ, Hersh PS, Kenyon KR (eds.). Eye Trauma. St. Louis: Mosby; 1991.
11. Keil J, Chen S. Contusion injuries and their ocular effects. Clin Exp Optom 2001;84(1):19-25.
12. Kaiser PK, Friedman NJ, Pineda R. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology, 2nd ed. Philadelphia: Saunders; 2004.
13. Pavan-Langston D. Manual of Ocular Diagnosis and Therapy, 4th ed. Boston: Little, Brown and Company; 1996.

