 |
Over the past few months, three companies announced their second pivotal Phase III FDA trial results for novel ocular surface disease agents, and the results aren’t just promising—they are exciting, for clinicians and patients alike. All trials met their pre-specified primary endpoints, meaning they could get approved within the next year. It’s valuable for us to know about these potential pharmaceuticals so we can begin identifying patients with certain ocular diseases and give them hope that new agents may come available in the near future to treat their condition.
 |
|
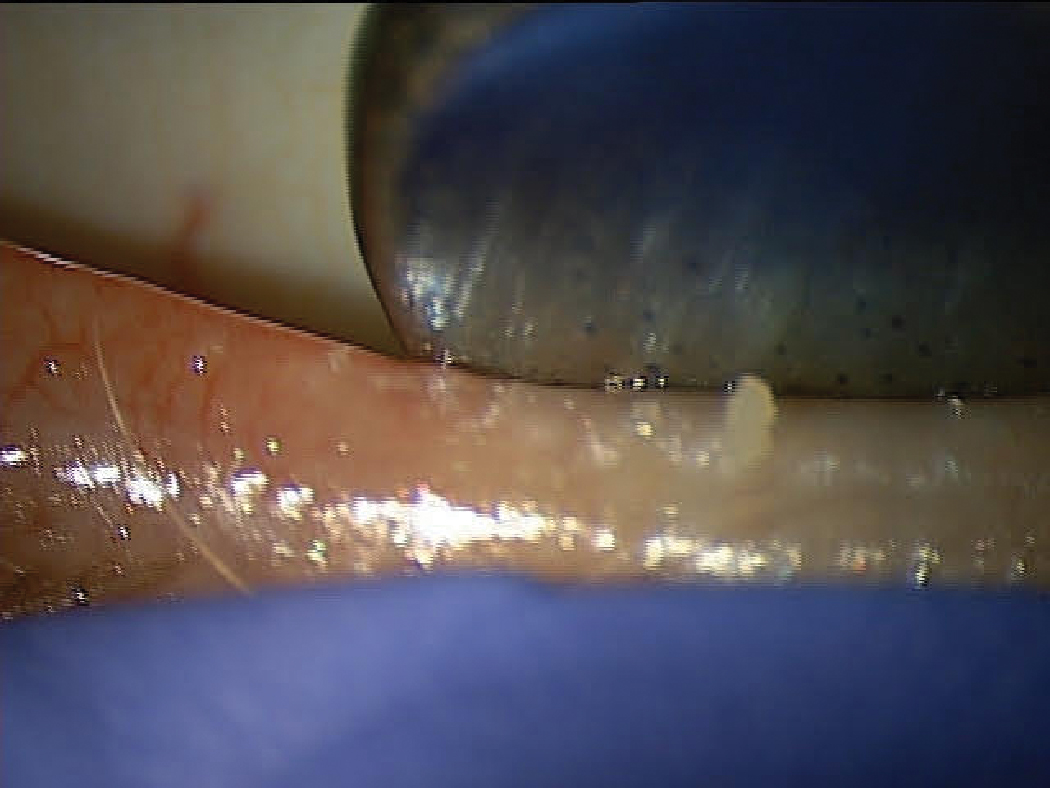
A patient with DED associated with MGD showing poor MG expression. Click image to enlarge. |
Perfluorohexyloctane: NOV03
This may be the first drug approved for the signs and symptoms of dry eye disease (DED) associated with meibomian gland dysfunction (MGD). Studies have shown that MGD is present in 86% of all DED cases.1 Most if not all current eye drops are 30µL to 50µL doses, but NOV03 is only 12µL—and that small amount goes a long way. It appears to practically replace a dysfunctional lipid layer and has been shown to last over four hours after a single administration. To put that in perspective, most therapeutic drops are measurable on the eye for about three to five minutes.
The second Phase III clinical trial results matched the previous study and revealed that NOV03 easily met both primary efficacy endpoints: statistical improvement in total corneal fluorescein staining and eye dryness score (using a visual analog scale), compared with hypotonic saline at day 57. Hypotonic saline is often a base for artificial tears. More impressive is the fact that these two endpoints were met as soon as two weeks. The drop is very comfortable and the only adverse event that occurred in more than 1% of patients was mild blepharitis. Even though the agent remains on the eye for four to six hours, patients did not report blurred vision. The drop is preservative-free and the study was dosed at QID.
Managing meibomian gland dysfunction in the most common form of DED, affecting upwards of 30 million patients, is an incredible need as current therapeutics are only addressing the inflammation that is a result of the MGD (or blepharitis), but do not treat the cause. This is a drug I am confident will give great hope to patients struggling with evaporative DED.
 |
|
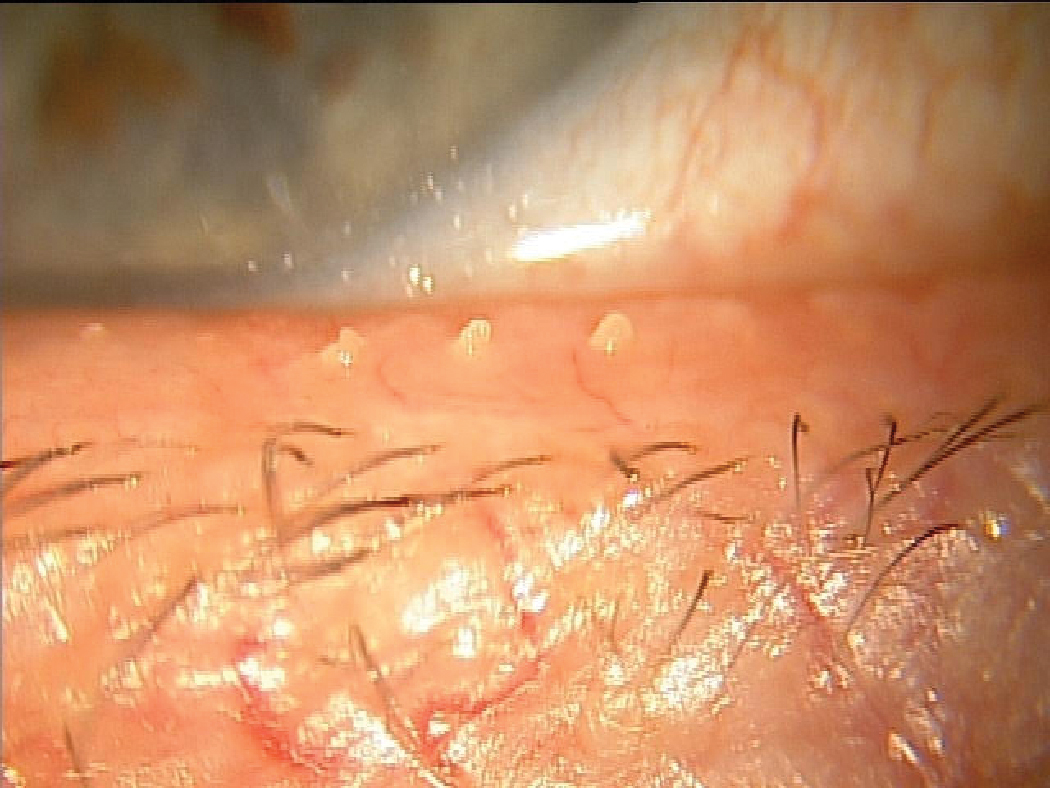
Another MGD patient that likely suffers from chronic tear instability resulting from inadequate lipid release to the ocular surface. Click image to enlarge. |
Lotilaner: TP-03
We’ve all seen our fair share of Demodex blepharitis, a condition estimated to affect about 25 million patients in the United States alone.2 Even that sizable number may be an underestimation. If doctors begin routinely looking more closely for collarettes—by having the patient look down while at the slit lamp and examining the base of the upper lid margin—an even greater number of patients will be identified.
Demodex is a common cause of itching, grittiness and eye dryness that leads to lash thinning, lash loss, evaporative dry eye, hordeola, chalazion, meibomian gland atrophy and rosacea.
Lotilaner (Tarsus Pharmaceuticals) is an anti-parasitic that works by paralyzing the mite’s nervous system through parasite-specific GABA inhibition. Tarsus’s results of their second Phase III pivotal trial are consistent with their previous Phase III trial for the treatment of Demodex blepharitis. TP-03 met the primary endpoints of collarette cure rate and mite eradication as well as all secondary endpoints, with high statistical significance. The study found that 89% of patients achieved a clinically meaningful collarette cure rate (<10 collarettes per eye), which is impressive considering there are few effective treatments for Demodex blepharitis, and no patients were permitted to scrub their eyelid margins. Further, 56% of patients showed a complete collarette cure rate and 31% showed a complete resolution of erythema.
Considering this is not a steroid or anti-inflammatory agent, the improvement in erythema is notable. The most common adverse event, which occurred in less than 10% of patients, was burning upon instillation. The drug was dosed at BID for six weeks and long-lasting effects are expected following this treatment duration.
Demodex is currently the most difficult form of blepharitis to manage, making it the most common blepharitis. A fast-acting, effective agent dosed for only six weeks is what this extremely large patient base is in desperate need of.
 |
|
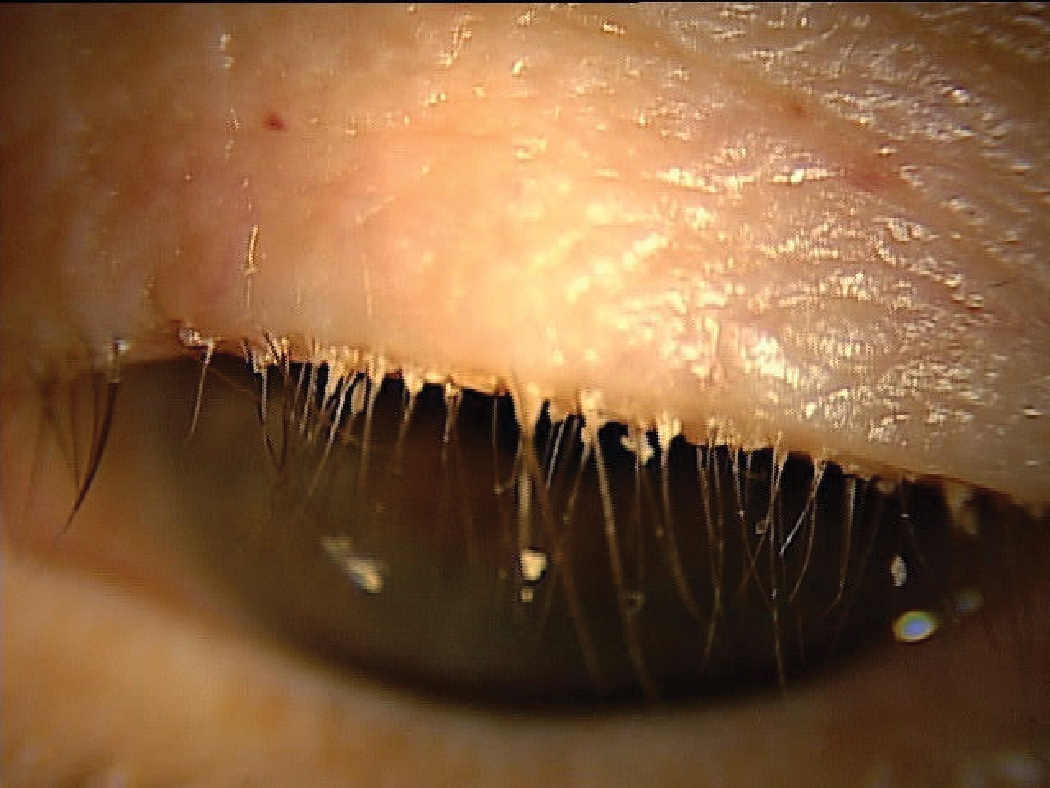
Having a patient look slightly down identifies collarettes in Demodex blepharitis. Click image to enlarge. |
Reproxalap
Inflammation is central to the development and perpetuation of dry eye. A pro-inflammatory mediator called reactive aldehyde species may play a key role in clinical manifestations of DED, and a new drug targets such activity.
Reproxalap (Aldeyra Therapeutics) is a small-molecule reactive aldehyde species (RASP) inhibitor. RASP has been shown to be greatly elevated in ocular (DED and allergic conjunctivitis) and systemic inflammatory diseases.3 Its mechanism of action works at a very high level in the inflammatory cascade, possibly positioning this drug at or above the level of where corticosteroids work, but without the potential of intraocular pressure elevation or other complications.4
I believe we are in great need of a fast acting, potent but safe, multi-modal anti-inflammatory agent that targets the direct pathway to cytokine production as inhibition of RASP in DED and potentially other ocular surface diseases, especially to patients who can’t take corticosteroids. The overlap between DED and allergic eye diseases may affect over 30 million people, let alone those with DED in isolation.
In the second Phase III FDA trial, data reached statistically significant improvements over vehicle for its pre-specified primary endpoints of Schirmer test scores and >10mm Schirmer responders. Both are FDA-approvable endpoints for DED. These data points were achieved after a single day of dosing, although data submitted will include trial results over five studies involving over 1,700 patients, including 12-week dosing studies. Those clinical trials also achieved statistically significant positive results for ocular dryness symptom score and ocular redness.
Exciting Times Ahead
These three drug candidates couldn’t be more desperately needed by patients, as they are far different from anything currently available. While we wait for them to be approved, we can begin identifying patients, educating them about what’s to come and provide hope and excitement for the next potential wave of OSD therapeutics.
Dr. Karpecki is medical director for Keplr Vision and the Dry Eye Institutes of Kentucky and Indiana. He is the Chief Clinical Editor for Review of Optometry and chairman of the affiliated New Technologies & Treatments conferences. A fixture in optometric clinical education, he provides consulting services to a wide array of ophthalmic clients. Dr. Karpecki’s full disclosure list can be found here.
1. Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-8. 2. Zhap YE, Wu LP, Hu L, Xu JR. Association of blepharitis with Demodex: a met-analysis. Ophthalmic Epidemiol. 2012;19(2):95-102. 3. Clark D, Karpecki P, Salapatek AM, et al. Reproxalap improves signs and symptoms of allergic conjunctivitis in an allergen chamber: a real-world model of allergen exposure. Clin Ophthalmol. 2022;16:15-23. 4. Higdon A., Diers AR, Oh JY. et al. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem J. 2012;442(Pt 3):453-64. |

