 |
A 56-year-old Hispanic male presented with a two-week history of “blurry vision” in his left eye. He described a cloudiness and fogginess to his vision and a left-sided dull, achy eye pain. Upon further questioning, he endorsed a three-week history of flashes and floaters in the left eye. The right eye was asymptomatic and he denied any similar previous episodes in either eye. His past ocular, medical and social histories were unremarkable. He had no known drug allergies and did not remember his last eye exam.
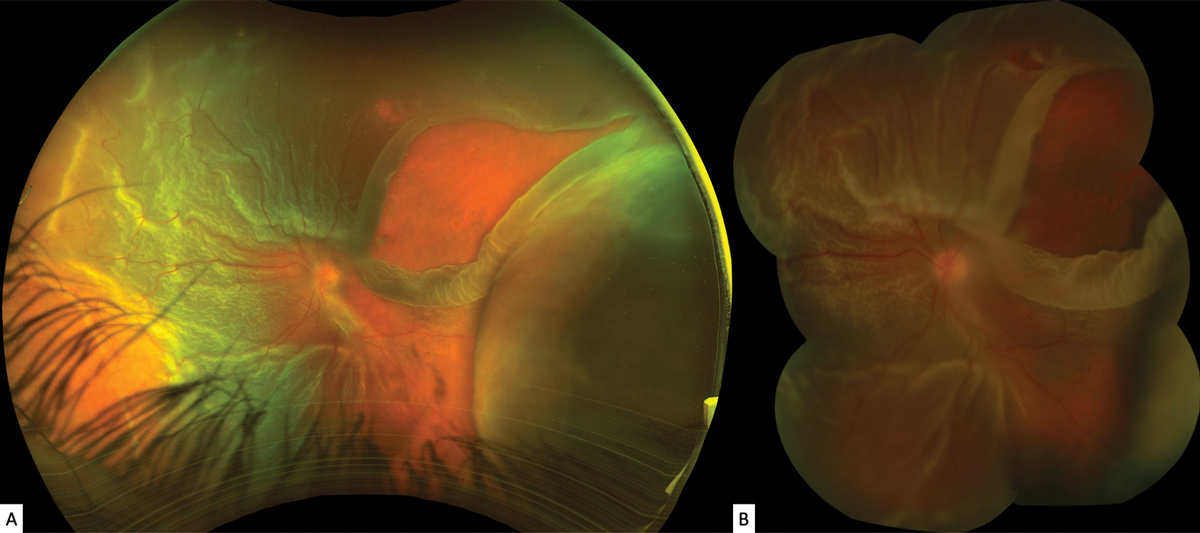
On examination, his best-corrected visual acuity (BCVA) was 20/25-1 OD and hand motions OS. There was a relative afferent pupillary defect in the left eye. His extraocular motilities were full OU, and there was a significant constriction to his confrontation visual fields OS. His intraocular pressure (IOP) measured 18mm Hg OD and 4mm Hg OS. Anterior segment examination revealed a deep anterior chamber (AC) OU with rare cells and 1+ flare OS. Imaging of the posterior segment is shown below (Figure 1).
 |
|
Fig. 1.Optos ultra-widefield (A) and Topcon (B) montage fundus images of the left eye. Click image to enlarge. |
Take the Retina Quiz
1. Which is seen in the fundus photo?
a. A retinal tear or retinal detachment (RD).
b. A choroidal detachment.
c. Pre-retinal membranes.
d. All of the above.
2. Which of the following is true of the membranes in the fundus photos?
a. They are secondary to retinal pigment epithelial cell proliferation.
b. They are secondary to chronic retinal ischemia.
c. They are fibrovascular in origin.
d. They can be treated with intravitreal anti-VEGF therapy.
3. What is the strongest preoperative risk factor for surgical failure of RD repair?
a. Acute macular involvement.
b. Proliferative vitreoretinopathy (PVR).
c. Vitreous hemorrhage.
d. None of the above; there is a 100% success rate of RD repair.
4. Which of the following is true of rhegmatogenous retinal detachment (RRD) repair?
a. Smaller-gauge surgical instrumentation yields a better prognosis.
b. Intravitreal steroid use yields a better prognosis.
c. Cigarette smoking yields a poorer prognosis.
d. Perfluoropropane (C3F8) gas always yields a poorer prognosis than silicone oil.
5. What level of VA will most patients achieve in the setting of postperative anatomic success?
a. Approximately 20/50 BCVA.
b. Ambulatory vision (approximately 20/800).
c. Light perception.
d. No light perception.
For answers, see below.
 |
|
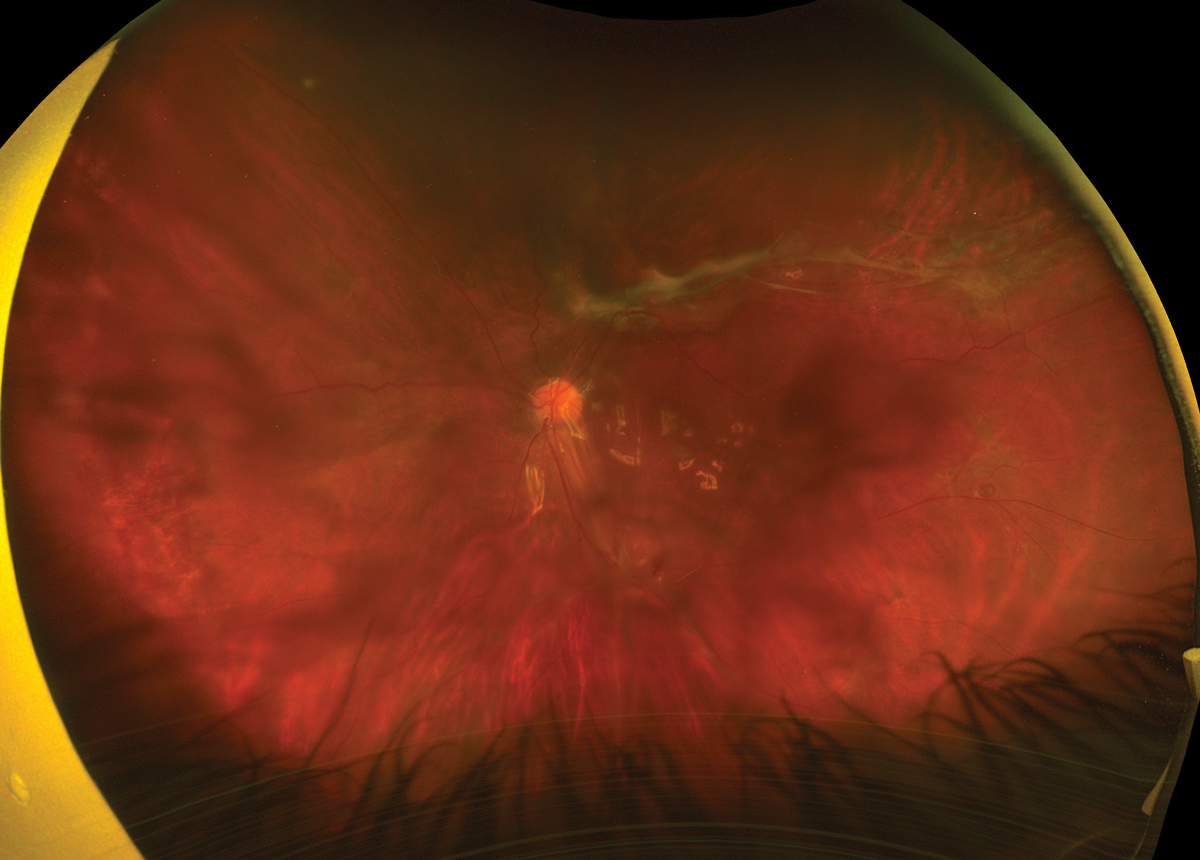
Fig. 2. Ultra-widefield fundus image of the left eye at post-op month two. Click image to enlarge. |
Diagnosis
Slit lamp examination of the patient revealed positive Shafer sign in the left eye with pigment clumping in the inferior vitreous and moderate vitreous haze. Fundus exam revealed a large radial superotemporal retinal tear with an adjacent smaller break at 12:30 in the left eye, with bullous surrounding subretinal fluid (Figure 1). The macula was detached, and there were diffuse preretinal tractional membranes anterior and posterior to the equator. There remained only a small sector of attached retina inferotemporally (Figures 1B and 2). Additionally, there were serous choroidal effusions nasally and temporally (Figure 1).
We diagnosed an RRD with PVR OS. The size and chronicity of the large retinal tear led to secondary hypotony with choroidal effusions, AC inflammation and ciliary body shutdown—resulting in the patient’s eye pain.
Discussion
PVR is defined as the avascular proliferation and contraction of fibrocellular retinal membranes associated with a rhegmatogenous retinal detachment.1,2 Despite surgical advances, PVR remains the leading cause of rhegmatogenous retinal detachment (RRD) surgical failure at a rate of 5% to 10% (mean of 7%).1-4 Risk factors for PVR development include retinal tear size, presence of vitreous hemorrhage, chronic intraocular inflammation, longstanding untreated RRD, unsuccessful RRD repair and penetrating ocular trauma.1,3-6 While its role is not completely understood, smoking is the only identified modifiable risk factor for PVR development.7
Disruption of the blood-retinal barrier--—as in the case of a retinal break or detachment, uveitis, cryotherapy and trauma—allows for the influx of inflammatory and retinal pigment epithelial (RPE) cells into the vitreous cavity.5,6 Once liberated, the proliferation of these cells leads to formation of preretinal and subretinal membranes.2 Proliferative membranes, once formed, contract and induce retinal traction.2 The tractional forces exerted from these membranes can create secondary retinal breaks as well as reopen breaks that have already been treated.2 These secondary breaks in the retina serve as a conduit for further influx of catalytic RPE and inflammatory cells.2 As demonstrated, the pathogenesis of PVR is cyclic and self-propagating.
Clinically, the retina takes on an opaque appearance with wrinkles and fixed folds.2 Due to gravity, PVR tends to begin inferiorly with fine membranes bridging the retinal folds as they contract. Often, one can appreciate proliferative membranes at the leading edge of a retinal break, which causes stiffening of the torn retina and a rolled posterior edge. Dynamic ultrasonography remains a powerful tool for identifying advanced PVR by evaluating the mobility and rigidity of the retina, which can aid prognostication and surgical planning.5 Retinal rigidity can also be assessed during scleral depression, and by evaluating retinal mobility during saccades on indirect ophthalmoscopy.1,5
Early PVR is characterized by vitreous haze, pigment clumping and retinal folds, and advanced PVR became further subdivided in a more recent classification after surgical success rates were better for posterior than anterior PVR.3,4 Many clinicians still simply grade PVR as mild, moderate or severe and acknowledge anterior/posterior extent of starfolds and traction.
Treatment Options
Surgical intervention remains the primary treatment modalities for these complex eyes.8 Pars plana vitrectomy (PPV) has been performed using 20-, 23-, 25- and 27-gauge instrumentation with equivalent outcomes.3 A thorough vitrectomy with complete removal of the vitreous and any preretinal membranes is necessary to achieve optimal outcomes.3 Comparison of intraocular tamponades revealed that silicone oil and perfluoropropane (C3F8) gas are both equivalent to each other but superior to sulfur hexafluoride (SF6) gas.9,10 Subgroup analysis, however, suggested better visual prognosis with silicone oil for eyes with anterior PVR.10
Unfortunately, the prognosis for these complex RRDs remains poor despite best surgical efforts. The retinal redetachment rate can be as high as 75% depending on preoperative severity.8 Reported anatomic success rate in these cases is 60% to 80%.8 Of those that are anatomically successful, only 40% to 80% will recover ambulatory vision (5/200) or better.6,8
The patient in question was referred to our retina service. Understanding the risks, benefits and alternatives to treatment, the patient underwent 25-gauge PPV, endoscopic laser photocoagulation, membrane peel, fluid-air exchange, cryotherapy and insertion of 5000-centistoke silicone oil.
On the first day of post-op, the patient’s vision was counting fingers at three feet and IOP was 4mm Hg. There was good silicone oil fill, and the retina was grossly flat under the oil with fresh laser surrounding the superotemporal retinal breaks. The patient was then lost to follow-up until month two of post-op, at which his vision remained at counting fingers and IOP was 17mm Hg. The retina remained attached under oil with an epiretinal membrane along the inferior arcade inducing traction (Figure 2).
Retina Quiz Answers
1) d; 2) a; 3) b; 4) c; 5) b.
Dr. Aboumourad is an assistant professor of ophthalmology at Baylor College of Medicine in Houston. He also practices at the Jamail Specialty Care Center of the Alkek Eye Center. He has no financial interests to disclose.
Dr. Dunbar is the director of optometric services and optometry residency supervisor at the Bascom Palmer Eye Institute at the University of Miami. He is a founding member of the Optometric Glaucoma Society and the Optometric Retina Society. Dr. Dunbar is a consultant for Carl Zeiss Meditec, Allergan, Regeneron and Genentech.
1. Yanoff M, Duker JS. Ophthalmology. 5th ed. Elsevier; 2018. 2. Pastor JC. Proliferative vitreoretinopathy: an overview. Surv Ophthalmol. 1998;43(1):3-18. 3. Idrees S, Sridhar J, Kuriyan AE. Proliferative vitreoretinopathy: a review. Int Ophthalmol Clin. 2019;59(1):221-40. 4. Coffee RE, Jiang L, Rahman SA. Proliferative vitreoretinopathy: advances in surgical management. Int Ophthalmol Clin. 2014;54(2):91-109. 5. Schachat AP, Wilkinson CP, Hinton D, et al. Ryan’s Retina. 6th ed. Elsevier;2018. 6. Pastor JC, de la Rúa ER, Martı́n F. Proliferative vitreoretinopathy: risk factors and pathobiology. Prog Retin Eye Res. 2002;21(1):127-44. 7. Xu K, Chin EK, Bennett SR, et al. Predictive factors for proliferative vitreoretinopathy formation after uncomplicated primary retinal detachment repair. Retina. 2019;39(5):1488-95. 8. Sadaka A, Giuliari GP. Proliferative vitreoretinopathy: current and emerging treatments. Clin Ophthalmol. 2012;6:1325-33. 9. Abrams GW, Azen SP, McCuen BW, et al. Vitrectomy with silicone oil or long-acting gas in eyes with severe proliferative vitreoretinopathy: results of additional and long-term follow-up: Silicone Study Report 11. Arch Ophthalmol. 1997;115(3):335-44. 10. Diddie KR, Azen SP, Freeman HM, et al. Anterior proliferative vitreoretinopathy in the Silicone Study: Silicone Study Report number 10. Ophthalmology. 1996;103(7):1092-9. |

