Masquerading features of various retinal and chorioretinal disorders can result in a diagnostic conundrum. By familiarizing oneself with the differentiating nuances of these findings, we can overcome many of these clinical challenges. This article demonstrates a few examples of such cases and presents a series of self-test questions to sharpen your diagnostic skills.
|
|
CASE #1:
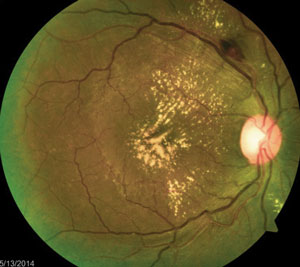
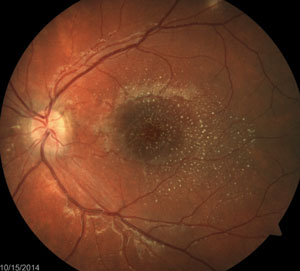
The above fundus photographs depict two separate patients, each complaining of blurred vision for a short but indeterminate length of time.
Both are new to your practice and have never had an eye exam before.
Questions
1. Which systemic processes may be involved in each patient presentation?
a. The patient on the left has poorly controlled diabetes, hypertension or a mix of both. The patient on the right may have a subclinical systemic infection.
b. The patient on the left has poorly controlled diabetes, whereas the patient on the right has high blood pressure.
c. The patient on the left has a branch vein occlusion, while the patient on the right has increased intracranial pressure.
d. Both patients have no systemic disease related to their ocular findings.
2. What does not need to be included in the differential diagnosis for the patient on the right, who has no prior medical history and normal vital signs?
a. Bartonella henselae
.
b. Acute viral illness.
c. Sarcoidosis.
d. Punctate inner choroidopathy.
3. Assuming the patient on the right is a 10-year-old with no significant past medical history, what is the best course of action?
a. Intravitreal dexamethasone.
b. Intravitreal anti-VEGF.
c. Topical third-generation quinolone antibiotic.
d. Short course of oral azithromycin.
4. You determine that the patient on the left has not been compliant with his antihypertensive medications. What do you do next?
a. Observe closely after counseling.
b. Contact patient’s primary care provider.
c. Treat with topical steroids and NSAIDs.
d. Prescribe oral steroids.
Answers
1) a; 2) d; 3) d; 4) b
Diagnoses
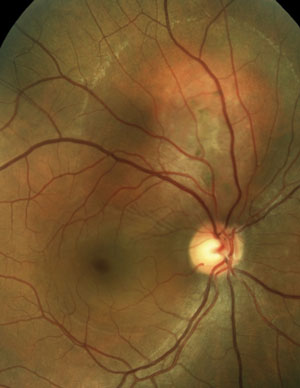
The patient on the right has hypertensive retinopathy whereas patient on the left has neuroretinitis caused by seropositive Bartonella henselae (cat scratch disease).
Discussion
Exudates appear in the intra- or subretinal space when the blood/retina barrier is interrupted within the retinal and/or choroidal circulation. The etiology of the vascular breakdown is wide-ranging and potential mechanisms include vascular disorders, such as hypertension or diabetes, as well as inflammatory and infectious diseases (see “Possible Causes of Retinal Exudates,” below).
In each of these conditions, the pattern of exudate—or lipid deposits resulting from leakage—may be different. For example, the two common conditions resulting in a so-called “macular star” exudate are hypertensive retinopathy and neuroretinitis. In other conditions, the exudate may be noted in the localized region of disease involvement, such as around aneurysms or along the involved vessels in a vein occlusion.
In neuroretinitis (right image), the exudates tend to have a perivascular distribution, located surrounding blood vessel terminations. Therefore, by combining the patient’s history (including existing medical conditions) and clinical presentation, you can develop a focused differential diagnosis and investigative process by which the cause, and the course of treatment, is determined.
Some investigators have speculated that a macular star is formed by transudation from capillaries deep in the optic nerve through the intermediate tissue of Kuhnt—a barrier that separates the optic nerve from the retina. Therefore, this type of exudative pattern is more common when there is both optic nerve and retinal (“neuroretinal”) involvement.
|
Possible Causes of Retinal Exudates
| |||||
| Vascular Disorders | Diabetic retinopathy |
Hypertensive retinopathy
|
Retinal vein occlusion
|
Coats’ disease
|
Retinal macroaneurysm
|
| Infectious Disorders |
Toxoplasmosis
|
Viral disease (e.g., herpes, HIV, CMV)
|
Syphilis
|
Bartonella
|
Tuberculosis
|
| Inflammatory Autoimmune Disease |
Sarcoidosis
|
Systemic lupus
erythematosus |
Posterior uveitic disorders
(multifocal choroiditis) |
Vogt-Koyanagi-Harada disease | |
| Miscellaneous | Choroidal neovascularization (any etiology) | ||||
CASE #2:
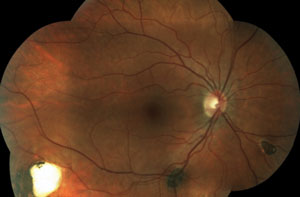
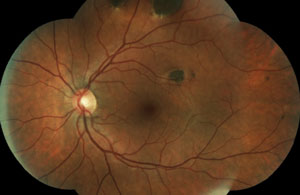
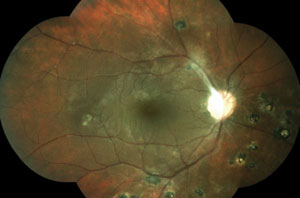
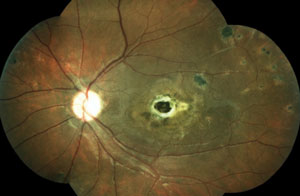
Two new patients come into your clinic. Both are accompanied by their entire immediate families. Both are 22 years old, and both are excellent historians. This is the first eye exam for each patient and neither one has any complaints. Their fundi are shown below.
|
|
|
|
Questions
1. Both patients are surprised by your exam findings, but only one family is shocked. This patient’s family has suffered through a string of relatives who had cancer diagnoses at a young age. Which patient has the strong family cancer history and what kind of cancer has her family suffered from?
a. Top patient, colorectal.
b. Bottom patient, lung.
c. Top patient, lung.
d. Bottom patient, colorectal.
2. Which patient has an underlying infectious etiology? What is the causative agent?
a. Top patient, histoplasmosis.
b. Bottom patient, toxoplasmosis.
c. Top patient, toxoplasmosis.
d. Bottom patient, histoplasmosis.
3. During your extensive counseling with the patient on the top, you recommend:
a. A six-week course of erythromycin by mouth.
b. Observe and return in six months to ensure no change.
c. Immediate referral to the appropriate gastroenterologist.
d. Referral for retina evaluation.
4. When explaining the diagnosis to the patient on the bottom, you decide to:
a. Start a six-week course of sulfamethoxazole/trimethoprim by mouth.
b. Refer for retina evaluation.
c. Refer to a pulmonologist.
d. Order serology studies to confirm the diagnosis.
Answers
1) a; 2) b; 3) c; 4) d
Diagnoses
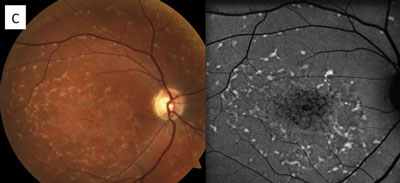
The patient in the top set of images has Gardner’s syndrome. The patient in the bottom set of images has toxoplasmosis.
Discussion
Pigmentary lesions of a nonchoroidal origin represent reactive changes occurring in the retinal pigment epithelium (RPE). Both hyperplastic and hypertrophic changes may result in any combination of increased pigmentation, increased number or increased size of the RPE cells. Toxoplasmosis causes these changes on either a congenital or acquired basis. Most occur from a single systemic infection, resulting in static lesions of varying size and significance to vision. However, the typical ocular findings in Gardner’s syndrome, a subtype of familial adenomatous polyposis, result in the progressive accumulation of lesions mimicking congenital hypertrophy of the RPE. The presence of an unusually high number of hypertrophic retinal pigment epithelial lesions (similar to congenital hypertrophy of retinal pigment epithelium [CHRPE]) and a typical teardrop appearance should clue the clinician in to a possible diagnosis of Gardner’s syndrome and the need for referral to a gastroenterologist.
While typical old, inactive non-macular toxoplasmosis lesions can be observed, patients with lesions involving the macula or of a significant size should be sent for a retina evaluation. So, the patient in this case (bottom images) has significant macular lesions and should be referred to a retinal specialist.
CASE #3:
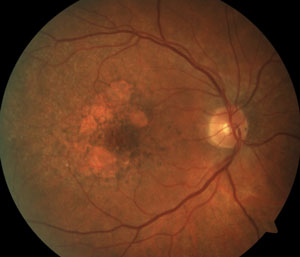
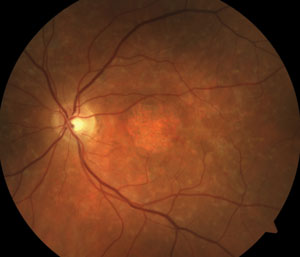
Two new patients come into your clinic with similar complaints of gradual loss of central vision. Both measure 20/200 centrally, and both report a decade of worsening. Both patients have smoked cigarettes for years.
Their fundus photos are shown below.
|
|
Questions
1. What is your best assessment based on the presented information?
a. Both have dry AMD.
b. Both have wet AMD.
c. The patient on the right has AMD and the patient on the left has macular dystrophy.
d. The patient on the left has AMD and the patient on the right has macular dystrophy.
2. Assuming one of these patients suffers from a macular dystrophy, which of the following is the most likely form?
a. Best disease.
b. Adult vitelliform.
c. Multifocal pattern dystrophy.
d. Sorsbys.
3. Which test is the least invasive, yet particularly beneficial to aid with the differential diagnosis in either patient?
a. Electroretinography.
b. Fundus autofluorescence imaging.
c. Genetic testing.
d. Fluorescein angiography.
4. For the patient on the left, which is the most significant counseling you need to provide?
a. Smoking cessation.
b. Genetic testing.
c. Recommend anti-VEGF therapy.
d. Annual eye health examination.
Answers
1) d; 2) c; 3) b; 4) a
Diagnoses
The patient on the right has atrophic AMD. The patient on the left has multifocal pattern dystrophy.
|
|
In fundus autofluorescent photography, drusen has a discrete blocking effect that causes reduced fluorescence. RPE atrophy does not fluoresce, so the degree and size of hypofluorescence or hypoautofluorescence depends on the extent and geographic area of RPE atrophy. Diseased RPE and lipofuscin cause hyperautofluorescence that may convert over time to hypofluorescence with the demise of the RPE.
|
| ||
Discussion
Fundus autofluorescence has become a valuable diagnostic tool for multiple retinal conditions, particularly in the management of patients within the spectrum of macular degenerative and dystrophic conditions. For example, in age-related macular degeneration, drusen as an early indicator for the disease shows subtle and varying degrees of hypo- or hyperautofluorescence, whereas the diseased retinal pigment epithelium (RPE) accumulating lipofuscin typically demonstrates hyperautofluorescence corresponding to the so-called “vitelliform” lesions.
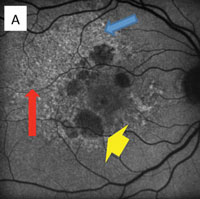
Eventually, the death of RPE (clinically known as geographic atrophy) represents the total absence of autofluoresnce (A).
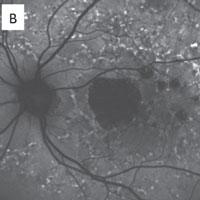
The RPE involvement in various macular dystrophies can have a distinctive appearance. For example, in multifocal pattern dystrophy (B), the RPE disease can be seen in a reticular pattern, whereas in fundus flavimaculatus these lesions are in the so-called pisciform (fish bone) pattern (C).
CASE #4:
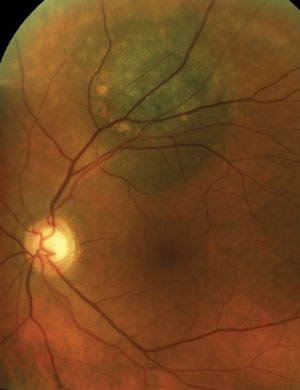
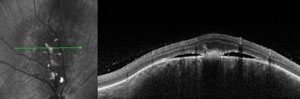
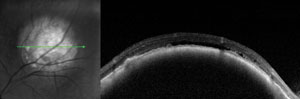
Two asymptomatic male patients were referred for evaluation of the lesions seen in the photos below.
Questions
1. Based on the OCT scans shown, what is the primary site of the lesions?
a. Inner retina.
b. Outer retina.
c. Retinal pigment epithelium.
d. Choroid.
2. Which of the following would you use to evaluate and diagnose these lesions?
a. Lesion’s height.
b. Presence of subretinal fluid.
c. Presence of orange pigment.
d. All of the above.
3. Which is the most appropriate referral for further management of these patients?
a. To patient’s primary care provider.
b. To a general ophthalmologist.
c. To an ophthalmic oncologist.
d. Follow-up only; no referral needed.
4. If the patient on the right elects to have treatment, which is the most likely plan?
a. Retinal photocoagulation.
b. Brachytherapy.
c. Enucleation.
d. Intravitreal anti-VEGF.
|
|
|
|
Answers
1) d; 2) d; 3) c; 4) b
Diagnoses
The patient on the left has a choroidal hemangioma. The patient on the right has a choroidal melanoma.
Discussion
Both of these patients have vision-threatening lesions that require urgent or emergent referral for evaluation by an ocular oncologist, but only one of the lesions is life threatening. Correctly identifying an ocular mass as a choroidal melanoma instead of a choroidal hemangioma involves adequate counseling and coordination with your collaborating ocular oncologist.
Recognizing high-risk features—such as the presence of orange pigment, subretinal fluid and change in tumor size—are crucial for differentiating a melanoma from a nevus.
Choroidal hemangiomas (left) are non-malignant vascular tumors that either exhibit an indolent course without growth or leakage or an aggressive course with growth and leakage. Diagnosis can be made with OCT, FA and B-scan. FA will show a distinctive, course, vascular pattern and B-scan will exhibit high internal reflectivity.
Choroidal melanomas (right) are the most common primary malignant intraocular tumor and they are missed or misdiagnosed daily. Indolent peripheral choroidal hemangiomas may be safely observed, while vision-threatening lesions are treated with anti-VEGF agents or PDT. With appropriate management, successful outcomes are common.
Once a diagnosis is made, correctly classifying the tumor (based on its ultrasonographic size into the small, medium and large categories described by the Collaborative Ocular Melanoma Study publications) can give patients a better idea of their chance of successful treatment and mortality rates.
Remember, essentially all primary choroidal melanoma metastases are to the liver, and routine surveillance with abdominal ultrasound or CT is required for detection of early metastatic disease after diagnosis and treatment.
Performing thorough exams and keeping your clinical skills honed are the keys to providing the level of care your patients deserve.
Dr. Rafieetary is a consultative optometric physician at the Charles Retina Institute in Memphis. Dr. Huddleston is an ophthalmologist in vitreoretinal fellowship also at the Charles Retina Institute. Dr. Sigler is a retina specialist at Ophthalmic Consultants of Long Island.
