Dry eye patients don’t share the same symptoms—some say “gritty,” while some say “stinging.” Likewise, they don’t all have the same signs—some have ocular inflammation or increased tear film breakup time, and some don’t.
But one thing they do have in common is tear film hyperosmolarity. (See “What is Hyperosmolarity?” below.) In fact, dry eye is defined (in part, at least) as hyperosmolarity of the tear film, according to the Definition and Classification Subcommittee of the International Dry Eye WorkShop (DEWS).2
And, neither signs nor symptoms always match with the results of diagnostic tests, such as tear-film break-up time, Schirmer tear testing or ocular surface staining.
As a result, researchers and clinicians have long been seeking a test that can objectively diagnose dry eye disease. Investigators have looked into optical coherence tomography, tear stress tests, fluorescein dye tracking with a xeroscope, and wavefront aberrometry as potential objective methods for diagnosing dry eye.
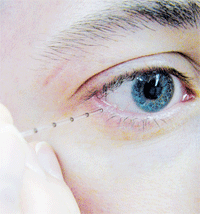
A micropipette is used to take a tear sample. Osmolarity testing has been declared the “gold standard” of objective dry eye diagnosis, and the single best marker of disease severity. Courtesy: Alisa Sivak, Centre for Contact Lens Research, School of Optometry, Univ. of Waterloo.
To that end, hyperosmolarity is the only characteristic of dry eye that can be objectively and reliably measured.4 Osmolarity testing has been declared the “gold standard” of objective dry eye diagnosis, and the single best marker of disease severity.5,6
Unfortunately, instruments to measure osmolarity have been restricted to research labs, and have been out of reach for workaday optometrists looking for reliable, in-office clinical testing. (See “Osmolality vs. Osmolarity”)
Osmolarity Testing
Osmolarity instruments are used to test all kinds of solutions—blood, serum, plasma, urine, bilirubin, milk, cell culture media, and many others. In the world of research, tears are almost among the least of these. Still, three different diagnostic technologies have been used for tear film osmolarity testing.
• Freezing point depression. The freezing point depression method of osmometry (i.e., measuring osmolarity) has been around for half a century. The two advantages of this method: It requires only a 0.2μL sample of tears (about 1/250th of a raindrop) and it’s highly accurate.
The freezing point depression method gets its name because the sample is supercooled to its freezing point. Water freezes at 0°C, but solutions (water + solutes) freeze at lower temperatures. (The freezing point of the solution is “depressed.”) So, the lower a solution’s freezing point, the higher its osmolarity. To calculate the osmolarity, the sample’s freezing point measurement is matched to known standards.
The problem with this method, at least for the average optometrist who wants to test dry eye, is that it’s expensive, technically challenging, and the equipment is typically used only by research labs.7
However, because of its accuracy, freezing point depression is the industry standard among the different methods of osmolarity testing.

• Vapor pressure. Compared to freezing point depression, vapor pressure osmometry is a relatively simple alternative method to measure tear osmolarity.8
This is the method I’ve been using for several years now. It operates on the principle that the vapor pressure of a solution is lower than the vapor pressure of the pure solvent (i.e., water) at the same temperature and pressure. In other words, the more particles that are present in the solution, the longer it takes to evaporate. Measuring this difference tells you the solution’s osmolarity level.
For this method, a sample is inserted into the instrument, where it is diffused onto a paper disk inside a small, enclosed chamber. As the sample evaporates, a sensor (a thermocouple hygrometer) measures the dew point temperature within the chamber. The difference between the dew point temperature and the baseline temperature is the dew point temperature depression. Calculating this difference provides the vapor pressure of the solution—that is, its osmolarity.
Although this method is quick (about 75 seconds) and reliable, it does have a big downside for measuring the tear film—it requires a relatively large sample (5μL) of tears. That’s a lot of tears when you consider that a normal person has about 7μL to 10μL of tears per eye, while a dry eye patient most certainly has less than that. Also, it requires skill to collect the sample without provoking the tear reflex, which will invalidate an accurate reading.

Have room in your office for one of these babies? A freezing point depression osmometer, like this one, is used only in research laboratories.
Only a few research studies have used this method of measuring osmolarity in dry eye disease.8 Accordingly, the National Eye Institute workshop on dry eye suggested that vapor pressure osmometry should be considered as a secondary dry eye diagnostic test.9
• Electrical impedance. This method takes advantage of the electrical conductivity of fluids to measure tear film osmolarity.
What is the “electrical conductivity of fluids”? All fluids (and all tissues, for that matter) have characteristic electrical properties. Indeed, any fluid (i.e., tears) can be characterized by its ionic content.10 So, if there’s any change in the composition and concentration of the ions within the tear fluid, then its electrical conductivity will change. This change can be correlated to its osmolarity measurement. (Blood glucose monitors use a similar technique.)
This is the method employed by the new TearLab osmometer (TearLab Corporation). Instead of a testing chamber, the TearLab osmometer uses a so-called “lab on a chip” at the tip of a handheld sampler. The “lab on a chip” is a single-use microchip embedded with gold electrodes that measure the electrical impedance of the tear fluid sample in a tiny channel in the chip.
To perform the test, the clinician uses the handheld sampler to collect a very small sample of tear fluid—just 50nL, an amount that’s “smaller than the period at the end of this sentence,” as the manufacturer describes it. The clinician places the tip of the handheld device adjacent to the inferior lateral meniscus of the tear film, and then the correct amount of fluid is absorbed onto the microchip by passive capillary action.

The electrical impedance osmometer takes an almost microscopic sample, just 50 nanoliters. Courtesy: TearLab Corp.
The handheld sampler is then docked into the stationary reader. Inside the handheld device, the gold-plated microchip measures the electrical impedance, and then the reader calculates and displays the osmolarity measurement in just a few seconds.
A recent paper compared the TearLab osmometer against the freezing point depression method (the industry gold standard) and determined that the TearLab method correlated well with the freezing point method.11
The investigators also described TearLab to be “effective as a single test for the discrimination of those with dry eye from those without the condition.” In addition, they concluded, “The patients with ‘dry eye’ in the present study were generally in the mild to moderate region of the diagnostic spectrum. If a technique is sensitive to such patients, its effectiveness is
validated because it is this group who are often difficult to diagnose.”
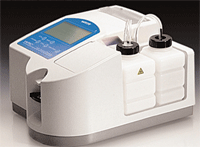
A vapor pressure osmometer is quick and reliable, but requires a very large sample of tears, making it rather impractical.
This method has a number of other advantages for the clinician in everyday practice. In addition to being quick, it’s relatively simple to administer (as long as the clinician learns to avoid the tear reflex), and it now has a dedicated CPT code (83861) so the test is reimbursable by Medicare (at $24 per eye) with no patient co-insurance or deductible.
On the downside, the instrument itself costs about $9,500 (including 80 test cards), which is up to $2,000 more than the cost of a vapor pressure osmometer. Also, at the moment, the instrument requires your office to be certified (Clinical Laboratory Improvement Amendments, or CLIA) as an approved “lab.” However, the manufacturer has applied for a CLIA waiver, so this may become a non-issue.
Test Results
Each of the above methods provides a numeric osmolarity measurement. The higher the measurement, the “drier” the eye. It’s measured in milliosmoles per liter (mOsmol/L), such that a reading of:
• 316mOsmol/L and higher indicates dry eye (hyperosmolarity).6 (I’ve taken readings as high as 360mOsmol/L, although I’ve heard anecdotally of a reading higher than 400mOsmol/L, which would be like shaking salt directly into your eye!)
• 290mOsmol/L to 316mOsmol/L suggests borderline or intermittent dry eye.
• 290mOsmol/L and below is healthy and normal.
With these numbers as a guide, one of the possibilities in dry eye treatment could be repeat osmolarity testing in order to track improvement in osmolarity scores over time.
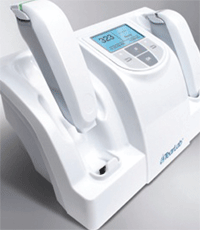
The microchip inside the handheld device measures the electrical impedance of the tears, then the stationary reader displays the osmolarity measurement in seconds.
Further Evaluation
Although osmolarity testing is diagnostic for dry eye, the only thing it tells you is that your patient has dry eye (with an indication to its severity). It cannot tell you the reason why the patient has dry eye—it can’t differentiate between aqueous deficient dry eye or evaporative dry eye. Additional clinical tests and subjective patient questionnaires are still necessary to enable you to treat the patient’s condition.
So, despite the objectivity of osmolarity testing, clinical judgment will remain an essential element in the assessment and treatment of dry eye.
Dr. Narayanan is an associate professor at the Pennsylvania College of Optometry at Salus University, Elkins Park, Pa. His area of research includes the tear film and ocular surface disease, with a special interest in the inflammatory component of dry eye disease. He has no financial affiliation with any of the products or companies mentioned.
1. Gilbard JP, Rossi SR. Changes in tear ion concentrations in dry-eye disorders. Adv Exp Med Biol. 1994;350:529-33.
2. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr;5(2):75-92.
3. Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004 Nov;23(8):762-70.
4. Khanal S, Tomlinson A, McFadyen A, et al. Dry eye diagnosis. Invest Ophthalmol Vis Sci. 2008 Apr;49(4):1407-14.
5. Farris RL. Tear osmolarity—a new gold standard? Adv Exp Med Biol. 1994;350:495-503.
6. Tomlinson A, Khanal S, Ramaesh K, et al. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006 Oct;47(10):4309-15.
7. Gilbard JP, Farris RL, Santamaria J 2nd. Osmolarity of tear microvolumes in keratoconjunctivitis sicca. Arch Ophthalmol. 1978 Apr;96(4):677-81.
8. Tiffany JM, Chew CKS, Bron AJ. Vapor pressure osmometry of human tears. Invest Ophthalmol Vis Sci. 1994 March 15; 35(4):S1692.
9. Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995 Oct;21(4):221-32.
10. Ogasawara K, Mitsubayashi K, Tsuru T, Karube I. Electrical conductivity of tear fluid in healthy persons and keratoconjunctivitis sicca patients measured by a flexible conductimetric sensor. Graefes Arch Clin Exp Ophthalmol. 1996 Sep;234(9):542-6.
11. Tomlinson A, McCann LC, Pearce EI. Comparison of human tear film osmolarity measured by electrical impedance and freezing point depression techniques. Cornea. 2010 Sep;29(9):1036-41.

