Ocular surface disease affects the majority of our patients in varying ways and to different degrees. By and large, it is not difficult to identify. We can see it at the slit lamp in the form of reduced tear lake, punctate staining and debris along the eyelid margin. We also see it in the faces of our patients when we ask them to describe how their eyes feel. Often, we can assess and manage dry eye with just a few standard tools and sufficient time and attention to the patient.
However, as diagnostic technology has improved our ability to differentiate the underlying causes of the many conditions that fall under the umbrella term of ‘ocular surface disease,’ we can now customize treatment more precisely, improving outcomes. We can also use more high-tech tools to document baseline status to quantify change over time and to convey to the patient the extent of their condition in hopes of getting their buy-in on efforts to control it. These are demonstrable benefits to both the patient and the practitioner. The question becomes: where does new technology fit into our practice and how can we use it to truly impact our patients’ treatment plan for the better? In this article, we’ll look at several newer concepts in meibomian gland assessment.
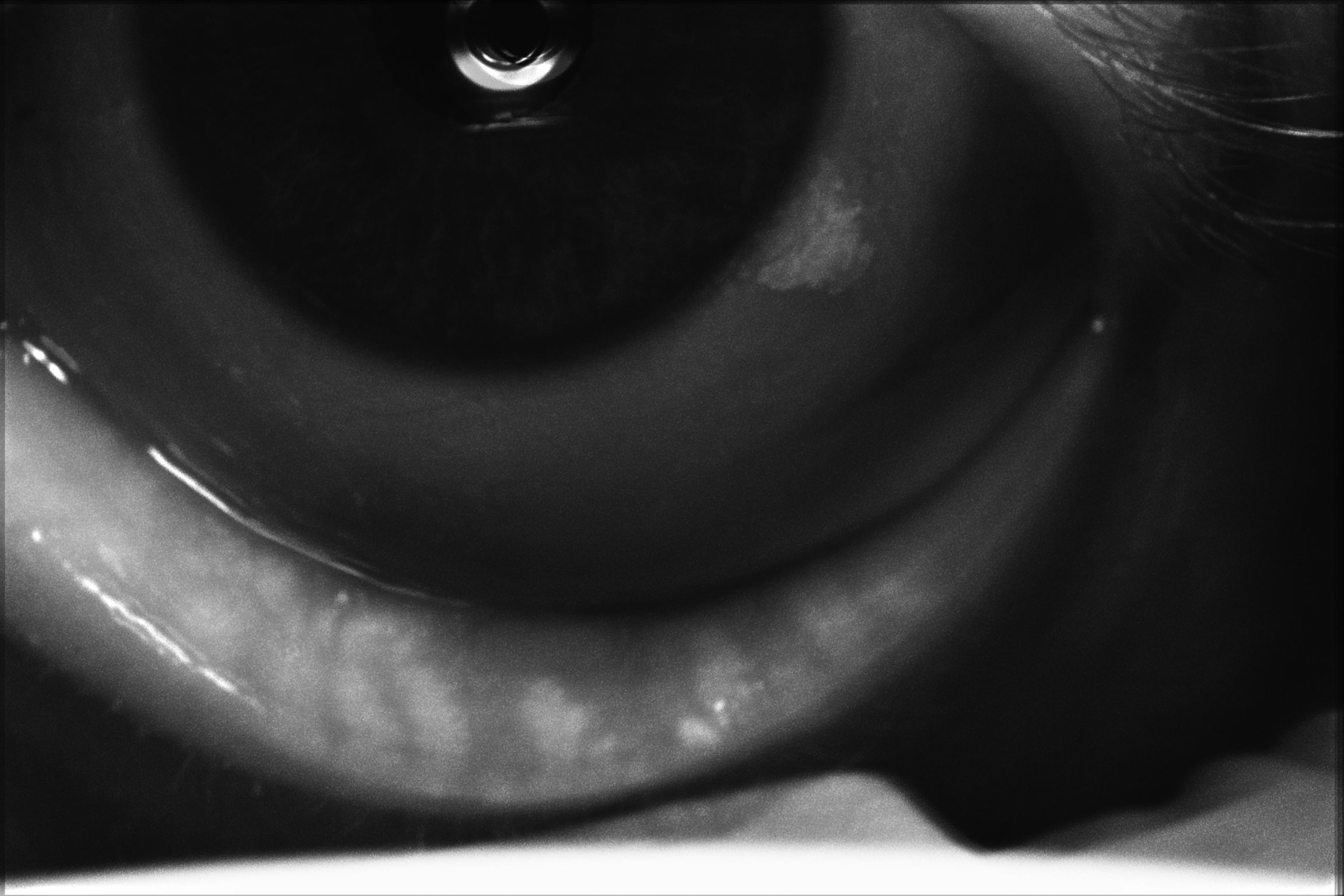 |
| Meibography of a patient with severe dry eye disease. Note they have moderate atrophy of the glands with moderate truncation and loss of gland structure. Click image to enlarge. |
Meibography
Secretions of the meibomian glands in both the upper and lower eyelids help to form the lipid layer of the tear film. Meibomian gland dysfunction (MGD) makes expression of these glands either difficult or near to impossible.1 Decreased lipid layer leads to tear film instability, resulting in evaporative dry eye.1 Patients experiencing poor meibomian gland function will have complaints that may include fluctuating vision, eyelid irritation and itching, and eyelids being sticky in the morning.2 MGD induces structural changes to glands and the eyelid margins that can lead to destruction of the meibomian gland orifices or atrophy.3
We typically evaluate the meibomian glands at the slit lamp, looking for evidence of irregular lid margins; hyperemia, telangiectasias, pitting or meibum orifice plugging. Inferior lids are much easier to assess and appreciate changes, but an attempt should be made to the upper glands as well. Digital pressure on the glands using a cotton tip applicator or Meibomian Gland Evaluator (Johnson & Johnson) can help evaluate the expressibility and quality of the meibum released. Meibum quality can be described as clear, cloudy, granular or inspissated, grading as follows:3,5
Grade 1: olive oil, clear
Grade 2: turbid, cloudy
Grade 3: cloudy with debris
Grade 4: toothpaste-like or inspissated
Historically, we have associated these slit lamp findings of expressibility and meibum quality with how we grade MGD. The more difficult it is to express the glands is thought to be a correlation to either gland atrophy or poor meibum quality. However, over the last few years with the advent and wider implementation of meibography, we are able have a direct view of the meibomian glands.
Meibography is specialized imaging that allows us to directly visualize the morphology and health of the meibomian glands with greater detail than slit lamp assessment alone. Using an infrared non-contact method, we can observe the meibomian glands in both the upper and lower eyelids, where we can see the true extent of the patient’s MGD. Normal glands appear straight and lined up like a white picket fence. Abnormal glands will appear tortuous, dilated, congested or atrophied.2,4
A retrospective study of meibomian gland structure changes using LipiScan (Johnson & Johnson) infrared meibography on the inferior central lid margin of 400 preoperative patients of cataract surgery age compared the level of atrophy (meiboscore) with physical findings on a slit lamp exam, including expressibility with meibum grade.5 The meiboscore grading system is as follows:
Grade 0: no loss of meibomian glands
Grade 1: 1% to 33% gland atrophy
Grade 2: 34% to 66% gland atrophy
Grade 3: greater than 66% atrophy
In this study, meibography revealed that 95.1% of patients had some level of gland atrophy.5 When comparing the slit lamp findings of expressibility (moderate and difficult) or a meibum grade 2 or worse in the same patients, there approximately 20% more identified as having atrophy via meibography.5 Further breakdown found a correlation between decreased expressibility of mebomian glands to increased mebomian gland atrophy but not between meibum quality and mebomian gland atrophy.1 Knowledge of both functional and architectural severity of MGD is important for clinical decision making, including appropriate ocular surface treatment and surgical decisions. Routine use of meibography would allow clinicians to identify patients with MGD earlier so we can treat and halt progression before extensive damage is done.4,6
Current available meibographers include Keratograph 5M (Oculus), Meibox and MX2 (Box Medical Solutions), IDRA, OSA and OSA Plus (SBM Sistemi), Ocu-Cam UltraHD (OICO), LacryDiag (Quantel Medical), LipiScan (Johnson & Johnson), and the HD Analyzer (Keeler). Soon, a new version of the iLux (Alcon) will be available that includes a built-in meibographer with the ability to take videos and photos along with providing thermal treatment for MGD.
Information from meibography, slit lamp exam findings and physical expression helps us to grade dysfunction of the meibomian glands and identify the type of dry eye and its underlying causes, allowing us to implement appropriate treatments. Patients with any grade of MGD would benefit from gland expression coupled with in-office lid exfoliation. In patients with MGD, blepharitis, Demodex and ocular rosacea, exfoliation of the eyelid at the lash line helps to remove the inflammatory biofilm that causes chronic lid disease and discomfort.4
With greater diligence to good lid hygiene, we can help to halt or slow the progression of MGD and improve patients’ symptoms.7 Some options on the market include eyelid exfoliation using BlephEx (Alcon) or LidPro (Mibo Medical) and heated expression treatment such as iLux (Alcon), LipiFlow (Johnson & Johnson) or TearCare (Sight Sciences), or even Mibo ThermoFlo (Mibo Medical) coupled with expression in the slit lamp.
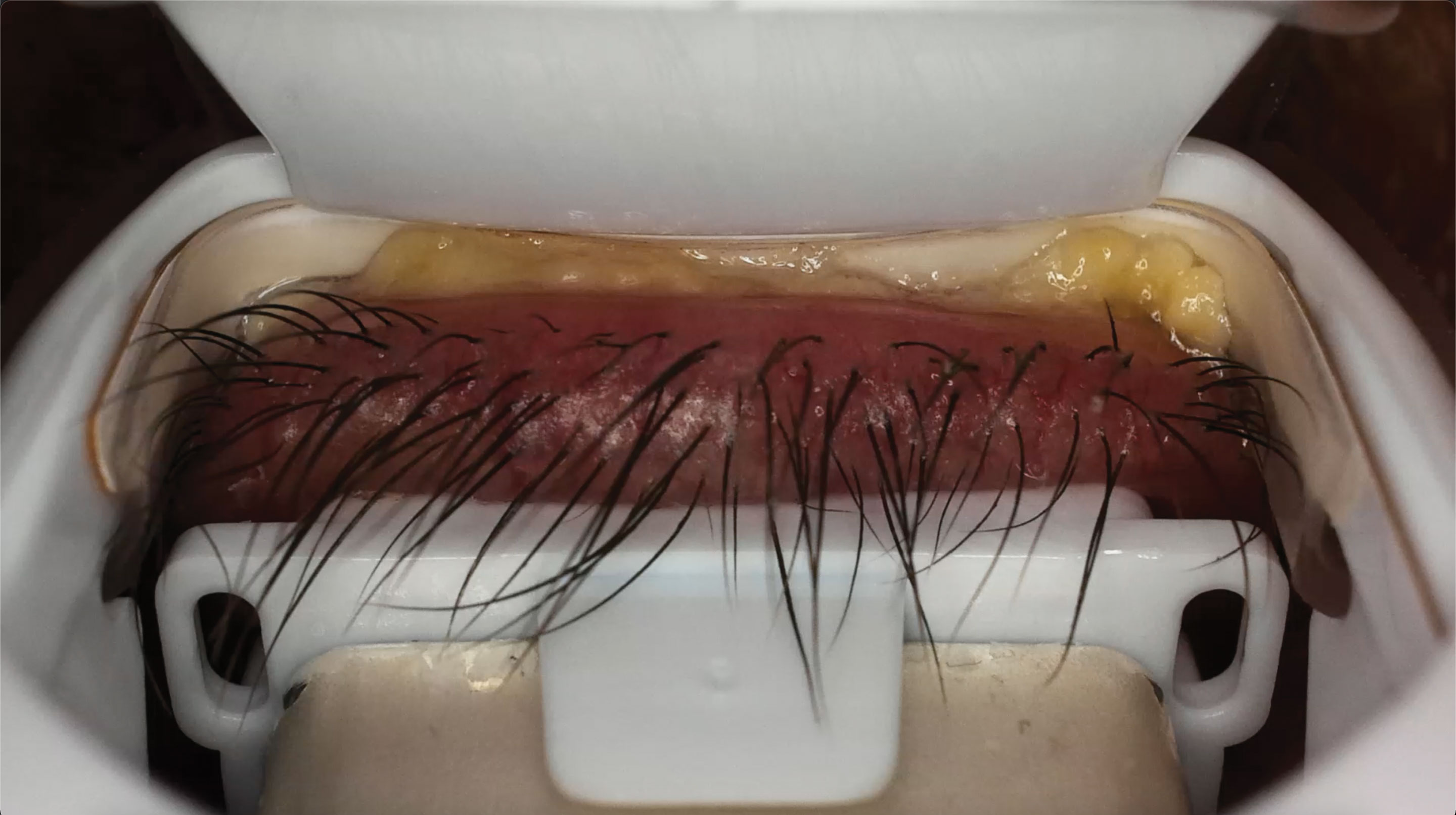 |
| This is the same patient undergoing iLux treatment. Note the significant amount of yellowy-white, poor quality meibum being expressed. Click image to enlarge. |
Interferometry
If meibography identifies MGD with greater sensitivity, its counterpart is interferometry, a tool that reveals how that dysfunction plays out on the ocular surface—again, in considerably more detail than our conventional tools.
Our earlier conception of a tri-layered tear film (lipid, aqueous, mucin) has since been updated to a model of an outer lipid layer overlying a mucoaqueous structure.8,9 If the tear film components become unbalanced, it can cause the entire system to fail, leading to dry eye. Impaired tear film stability is considered an essential criterion for diagnosing abnormality of tear film per the TFOS DEWS II report.6 As our concepts get more sophisticated and nuanced, so too should our techniques.
Tear film quality has most commonly been monitored using tear break-up time with application of sodium fluorescein, which in itself is known reduce the stability of the tear film.6 Non-invasive tear break up time (NIBUT) can be obtained from placido disc images reflected by the cornea from the tear film, and can be done with many of the current corneal topography systems.6 Other testing that can be performed to assess tear film quality includes thermography, Schirmer test, tear osmolarity, MMP-9 marker identification with InflammaDry (Quidel) and, though less readily available, tear film ferning.6
Another technique—a noninvasive one at that—to monitor tear film stability is interferometry, which analyzes both the tear film stability and thickness of the lipid layer in a semiquantitative method.9 Interferometry can distinguish clinical subtypes of dry eye by looking at the balance between the lipid and aqueous layers of the film.9 This test has been used in numerous studies of patients with different underlying cause of dry eye and has provided more information and understanding of the tear film itself.
A previous study found that patients with both non-Sjögren’s aqueous-deficient dry eye (ADDE) and MGD showed a very specific interferometric pattern that was different than those who were non-Sjögren’s ADDE or MGD only.8 Patients with only non-Sjögren’s ADDE had a shortened NIBUT, an increased lipid layer thickness and a reduced tear secretion. In comparison, patients with only MGD had thin lipid layer but sufficient tear secretion and a shortened NIBUT.8 This and other studies suggest that measuring these data points can potentially be used to diagnose and monitor dry eye disease.10-12
The LipiView II device (Johnson & Johnson) has both meibography and interferometry capabilities. Studies on the instrument’s ability in the MGD are well documented; however, the diagnostic contribution to DED is not yet established.6
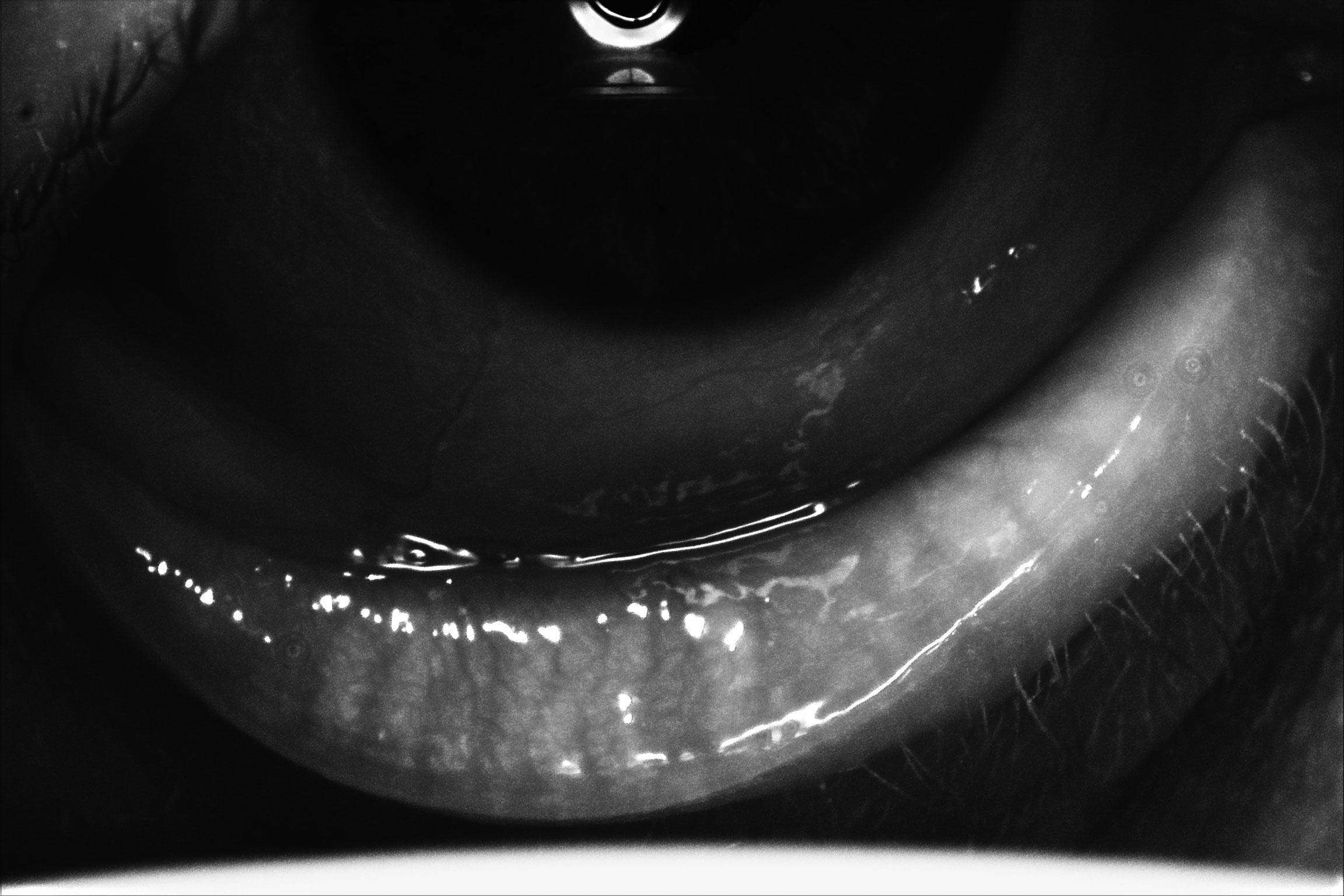 |
| Meibography of a patient with severe dry eye disease. Note they have moderate atrophy of the glands with moderate truncation and loss of gland structure, OS greater than OD. Click image to enlarge. |
Corneal Sensitivity
Altered corneal sensitivity is not a new concept, but testing for it is widely underused. As a result, affected patients often go undiagnosed.
In dry eye, corneal sensitivity increases with disease severity.6 In other conditions—most notably neurotrophic keratitis, surgery and trauma—sensitivity decreases. Testing corneal sensitivity can also help differentiate suspected herpes simplex keratitis from herpes zoster ophthalmicus.13 Although quantitative measurements are not required, with newer treatments for neurotrophic keratitis like Oxervate (cenegermin-bkbj ophthalmic solution 0.002%, Dompé), some insurers could begin to require this in order to cover these medications.
While you can use a cotton wisp or floss to test corneal sensitivity, dedicated instruments exist to more quantitatively measure this, such as the Cochet-Bonnet (Western Ophthalmics) or non-contact air-jet esthesiometers. The handheld Cochet-Bonnet has a thin, retractable nylon monofilament, and the length can be adjusted anywhere from 5mm to 60mm, allowing the user to apply varying pressure. As the length is decreased the pressure increases and the length is recorded.13
The non-contact esthesiometer is better in measuring lower stimulus thresholds than the Cochet-Bonnett.13 It assesses the corneal sensation threshold in an accurate and repeatable manner by measuring threshold sensitivity to a composite stimulus consisting of air pressure along with tear film evaporation and disruption.13
Tear Menicus Assessment
Located within the junction of the bulbar conjunctiva and eyelid margins, the tear meniscus is the fluid reservoir from which the precorneal tear film arises.6 Patients with dry eye may experience a decrease in the menisci.14 The easiest and most accessible way to measure it is via slit lamp exam using the beam; more high-tech methods include video-meniscometry and OCT meniscometry.6 In anterior segment OCT applications, the high-resolution imaging produces detailed cross-section images of the anterior segment.14,15 It can also gather information on tear meniscus height, depth and cross-sectional area measurements.16
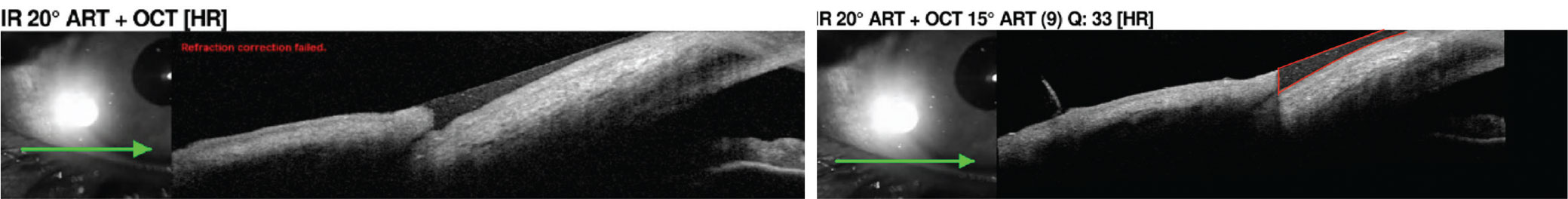 |
| Tear meniscus measurement using the anterior segment lens on OCT. The tear meniscus is outlined in red in the right image, which can be further evaluated for quality. Click image to enlarge. |
Visual Fluctuation
Patients with ocular surface disease, particularly dry eye, often experience poor or fluctuating vision due to instability of the tear film—the eye’s first (though admittedly least consequential) refractive medium. We typically elicit subjective reports of this phenomenon from the patient.
Those interested in gathering objective data can do so with the HD Analyzer (Visionmetrics/Keeler), which uses a laser diode to emit a light beam through the ocular media onto the retina; the light reflection is recorded by a camera. When there are irregularities in the air/tear film interface on the ocular surface, fluctuations in vision occur as light scatter.17 This scatter is measured by the system as degraded retinal image quality.
The device quantifies the effect using a metric called objective scatter index (OSI).18 The HD Analyzer measures changes in the OSI over a period of 20 seconds (taking one image every 0.5 second), with higher intraocular scatter correlating to higher OSI.17 Normal OSI score is less than 1.17 This is used to provide an analysis for the tear film quality and a concept called vision break-up time, which is the time it takes for a patient to lose one line of vision on the Snellen chart.17 This approach is most revealing in cataract patients, given the greater significance of the crystalline lens in the ocular media, but can reveal distinctive patterns of tear film refractive quality as well.
Argentinean ophthalmologist Roger Zaldivar identified three tear film quality patterns that correlate with vision break-up time: (1) ladder (a continuous increase of OSI), (2) seesaw (instability of OSI without improvement after blinking) and (3) plateau (steady high OSI).17 Plateau is normal, while the other two indicate abnormal tear film dynamics; the ladder pattern will have the highest OSI values.17
The HD Analyzer OSI scoring is also used to help determine whether a corneal or lens-based surgery is required.18 Additionally, it is able to perform high-definition images of the meibomian glands.18
How to Cover the Cost
When purchasing new technology, it’s important to make sure it is a good investment for the clinic. If the equipment sits idle or fails to provide pertinent usable information, it could be a drain on a practice’s resources. Currently, most advanced testing—similar to dry eye in-office treatments—is oftentimes reimbursed out-of-pocket. Many patients understand that having the right equipment and being able to provide a higher level of care can require additional expenses. Other times, we have to educate them on the importance of said testing so they see the added value it brings to their care.
Another thing to consider is the amount of reimbursement, even if you do decide to use the patient’s insurance. In either case, it’s good practice to have them sign an Advance Beneficiary Notice, which is a waiver of liability and should be given to the patient prior to providing the service. This ensures the patient is aware that the testing being given may or may not be covered and that they are financially responsible if it is not covered.
The CPT code 92285 is used for “external ocular photography with interpretation and report documentation of medical progress” and should be used for close-up photography, slit lamp photography, gonio photography, etc.19 It is a bilateral code and is reimbursable by Medicare for the photo and interpretation.19 These amounts are adjusted in each area by local wage indices.
Currently, the code 0330T is used for “tear film imaging, unilateral or bilateral with interpretation and report” and exists for tear film interferometry but is a Level III HCPCS code.20 This means that it is used to track utilization and is reported to the carrier, but there is no current reimbursement associated with it. Since this code is more specific, it is to be used instead of CPT code 92285 for this testing.20
Similarly, the code 0507T is to be used for “near infrared dual imaging (i.e., simultaneous reflective and transilluminated light) of meibomian glands, unilateral or bilateral, with interpretation and report” exists for meibography.20 This is also a Level III HCPCS code and, for data collection, it’s not reimbursable. Again, CPT code 92285 is not usable for this testing.
Regardless, if you decide to submit for reimbursement with the patient’s insurance, don’t forget to include your assessment in the patients plan or within their chart.
Is It Worth It?
Several of the advanced testing tools discussed here have more than one application—including some unrelated to dry eye. Those may be more obtainable and make more financial sense to add to your office’s armamentarium. More applications means more use, possibly with better reimbursement. Higher volume practices or those specializing in dry eye or corneal disease would likely have more use of the more singular use tests. Facilities that partake in research may also benefit from having multiple of these as well.
While we can’t have all the toys, there is no question as to the importance of the information that they can provide, even if some are more clinically relevant than others. It is important to look at your clinic, what technology is needed to help your patients now and what will help grow your practice into what you want it to be in the future.
Dr. Koetting practices at the secondary/tertiary surgical center Virginia Eye Consultants in Norfolk, VA. Her primary focus is ocular disease, specializing in anterior segment and corneal disease, neuro-optometry and perioperative care. She also participates in clinical research and the maintenance of the referral network alongside the practices other optometrists. Dr. Koetting serves as the externship director and is adjunct faculty for several schools and colleges of optometry. She is a consultant for Glaukos, Eyevance, Ivantis, Alcon, Orasis and IVL, as well as a speaker for Dompé, Glaukos, RVL and Eyevance.
1. Wise RJ, Sobel RK, Allen RC. Meibography: A review of techniques and technologies. Saudi J Ophthalmol. 2012; 26(4):349-56. 2. Foulks GN, Bron AJ. Meibomian gland dysfunction: A clinical scheme for description, diagnosis, classification and grading. Ocul Surf. 2003; 1(3):107-26. 3. Bron AJ, Benjamin L, Snibson GR. Meibomian gland disease: classification and grading of lid changes. Eye. 1991; 5(4):395-411. 4. Nichols K, Foulks G, Bron A, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011; 52(4):1922-9. 5. Yeu E, Koetting C, Calvelli, H. Understanding the prevalence of changes to meibomian gland architecture in the US cataract surgery population. ASCRS 2021. 6. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017; 15(3):539-74. 7. Lane S, DuBiner H, Epstein R, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31(4):396-404. 8. Willcox MDP, Argueso P, Georgiev GA, et al. TFOS DEWS II Tear Film Report. Ocul Surf. 2017;15:366-403. 9. Cher I. A new look at lubrication of the ocular surface: fluid mechanics behind the blinking eyelids. Ocul Surf. 2008;6:79-86. 10. Arita R, Morishige N, Fujii T, et al. Tear interferometric patterns reflect clinical tear dynamics in dry eye patients. Invest Ophthalmol Vis Sci. 2016; 57(8):3928-34. 11. Ibrahim OMA, Dogru M, Takano Y, et al. Application of visante optical coherence tomography tear meniscus height measurement in the diagnosis of dry eye disease. Ophthalmology. 2010; 117(10):1923–9. 12. Shen M, Li J, Wang J. Upper and lower tear menisci in the diagnosis of dry eye. Invest Ophthalmol Vis Sci. 2009; 50(6):2722–6. 13. Efron, N. Contact Lens Practice E-Book. United Kingdom, Elsevier Health Sciences, 2016. 14. Anuradha R, Dhasmana R, Nagpal RC. Anterior segment optical coherence tomography for tear meniscus evaluation and its correlation with other tear variables in healthy individuals. J Clin Diagn Res. 2016; 10(5):NC01-4. 15. Wolffsohn JS, Peterson RC. Anterior ophthalmic imaging. Clin Exp Optom. 2006; 89(4):205-14. 16. Mainstone JC, Bruce AS, Golding TR. Tear meniscius mesurement in the diagnosis of dry eye. Curr Eye Res. 1996; 15(6):653-61. 17. Gouvea L, Waring GO, Brundrett A, et al. Objective assessment of optical quality in dry eye disease using a double-pass imaging system. Clin Ophthalmol. 2019; (13):1991-6. 18. VanArsdale, E. Seeing dry eye differently with the HD analyzer OQUAS tear film module. www.blog.keelerusa.com/seeing-dry-eye-differently-with-the-hd-analyzer-oqas-tear-film-module/. Keeler USA. September 25, 2020. Accessed August 24, 2021. 19. Centers for Medicare and Medicaid Services. www.cms.gov/medicare/physician-fee-schedule/search?Y=0&T=4&HT=0&CT=3&H1=0507T&M=5. Accessed August 5, 2021. 20. Corcoran Consulting. www.corcoranccg.com. Accessed August 5, 2021. |

