As multidrug-resistant infections become more prevalent, we continually need improved antibiotics that are effective against these pathogens. Vancomycin resistance in pathogens such as Enterococcus is one of our most serious concerns. Weve generally regarded vancomycin as the gold standard for multidrug-resistant infections. When these infections become resistant to vancomycin as well, the prognosis for affected patients can be grim. Some reports estimate mortality rates to be more than 35%.1
For the first time in more than 30 years, however, we have a completely new antibiotic class available in the fight against multidrug-resistant pathogens. This class, known as the oxazolidinones, may one day become part of our therapeutic arsenal as well.
Five Mechanisms
Antibiotics generally work by disrupting five mechanisms:
Bacterial cell wall synthesis. Drugs that act upon bacterial cell wall synthesis include the penicillins, the cephalosporins, bacitracin and vancomycin.
Cytoplasmic membrane. Polymyxin B and gramicidin both affect the bacterial cytoplasmic membrane and disrupt the osmotic integrity of the cell.
Folic acid synthesis. Drugs that affect this intermediary metabolism include sulfonamides, pyrimeth-amine and trimethoprim.
DNA synthesis. The fluoroquinolones alone affect the bacterial DNA synthesis.
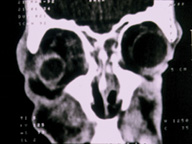
Orbital cellulitis is an ophthalmic indication for linezolid.
Protein synthesis. Drugs that affect protein synthesis act either upon the ribosomal 30S or 50S subunit. The aminoglycosides and tetracylines both bind to the 30S subunit, while the macrolides, chloramphenicol and clindamycin affect protein synthesis by binding to the ribosomal 50S subunit.2 Pathogens have demonstrated cross-resistance between these latter three medications.
The oxazolidinones also act on the ribosomal 50S subunit, although the exact mechanism of action for the oxazolidinones is controversial. The oxazolidinone mechanism is thought to be unique as there is no reported cross-resistance between oxazolidinones and other protein synthesis inhibitors.3
First in Its Class
The oxazolidinones were originally patented at DuPont in the late 1970s. The oxazolidinones are completely synthetic and are active against a large spectrum of Gram-positive bacteria, including methicillin- and vancomycin-resistant bacteria.
Researchers initially stopped testing drug candidates due to concerns about toxicity. Last year, however, Zyvox (linezolid, Pfizer) became the first FDA-approved oxazolidinone for clinical use.
Linezolid is indicated for skin and soft tissue infections and pneumonia caused by Gram-positive pathogens. Ophthalmic indications may include orbital cellulitis that is caused by multidrug-resistant pathogens or allergy to other antibiotics. Future studies are necessary to determine whether linezolid can cross the blood-aqueous-barrier to treat ocular infections such as endophthalmitis.
Linezolid is effective against Staphylococcus aureus, coagulase-negative staphylococci, enterococci, Streptococcus pneumoniae, and some anaerobic bacteria. It is consistently active against pathogens that are resistant to methicillin, penicillin and vancomycin.4 Susceptibility testing showed that linezolid had the most complete antibacterial spectrum against all staphylococci, streptococci and enterococci, when directly compared with vancomycin, levofloxacin, macrolides and clindamycin.5
Linezolid appears to be bacteriostatic toward staphylococci and bactericidal toward streptococci. It also shows potential activity against mycobacteria but has limited activity against Gram-negative bacteria and anaerobes.6
Its ability to penetrate well-perfused structures makes linezolid highly effective against post-surgical infections. Its clinical usefulness is enhanced by its ability to accumulate to high levels in the cerebrospinal fluid, osteoarticular tissues and synovial fluid. For example, linezolid has successfully treated Nocardia-related infections of the central nervous system.7 Although not FDA-approved for endocarditis, it has also successfully treated cases of endocarditis due to vancomycin-resistant enterococci.
Three Forms
Zyvox is available in tablets (400mg and 600mg), as an oral suspension (600mg) and intravenously (600mg). It is not currently available in an ophthalmic topical form. The recommended adult dose is bid for 10 to 28 days depending on the condition.
Linezolid is generally well tolerated. Adverse effects include diarrhea, nausea, headache, altered taste, rashes, reversible myelosuppression, increased liver enzymes and tongue discoloration. These effects are more likely to occur when treatment exceeds four to six weeks but should resolve upon discontinuation of the medication. Linezolid-induced nephropathy has been reported after extended treatment lasting more than six months.8
A few rare cases of linezolid-resistant pathogens have already been reported. To reduce the prevalence of resistance, practitioners must use this class of drug only when indicated and for the shortest amount of time to treat the infection.
While not currently available in ophthalmic topical formulations, the development of a new antibiotic drug class is very important to help fight the increasing numbers of drug-resistant infections. Hopefully, more research will be done in the future to develop ophthalmic oxazolidinones.
1. Brickner SJ. Hutchinson DK, Barbachyn MR, et al. Synthesis and antibacterial activity of U-100592 and U-100766, two oxazolidinone antibacterial agents for the potential treatment of multidrug-resistant gram-positive bacterial infections. J Med Chem 1996 Feb 2;39(3):673-9.
2. Yolton DP. Anti-infective Drugs. In: Bartlett JD, Jaanus SD, eds. Clinical Ocular Pharmacology. 4th ed. Boston: Butterworth Heinemann, 2001;11:219-47.
3. Diekema DJ, Jones RN. Oxazolidinone antibiotics. Lancet 2001 Dec 8;358(9297):1975-82.
4. Bozdogan B, Appelbaum PC. Oxazolidinones: activity, mode of action, and mechanism of resistance. Int J Antimicrob Agents. 2004 Feb;23(2):113-9.
5. Jones RN, Ballow CH, Biedenbach DJ, ZAPS Study Group Medical Centers. Multi-laboratory assessment of the linezolid spectrum of activity using the Kirby-Bauer disk diffusion method: report of the Zyvox Antimicrobial Potency Study (ZAPS) in the United States. Diagn Microbiol Infect Dis 2001 May-Jun;40(1-2):59-66.
6. Gemmell CG. Susceptibility of a variety of clinical isolates to linezolid: a European inter-country comparison. J Antimicrob Chemother. 2001 Jul;48(1):47-52.
7. Moylett EH, Pacheco SE, Brown-Elliott BA, et al. Clinical experience with linezolid for the treatment of nocardia infection. Clin Infect Dis 2003 Feb 1;36(3):313-8.
8. Corallo CE, Paull AE. Linezolid-induced neuropathy. Med J Aust 2002 Sep 16;177(6):332.

