 |
Proliferative retinal diseases are one of the leading causes of vision loss worldwide. Many treatments are aimed at reversing these conditions and preserving visual function. However, in order to do so, timely diagnosis and management is vital. As complications can be severe, more effective therapeutic solutions are continuously being evaluated.
It is important that the eyecare practitioner be able to accurately identify these treatable proliferative processes as early as possible. As such, understanding how and why they arise is crucial.
Normal Blood Supply
Proliferative retinal diseases are generally classified as either causing retinal or choroidal neovascularization. In order to understand their mechanisms, it is first necessary to distinguish normal retinal angiogenesis and blood supply. As we already know, for vision to occur, light needs to reach the photoreceptors. Because of this, the outer retina is largely avascular, as blood vessels would prohibit image formation if located immediately in front of the photoreceptors. Instead, the entire retina is nourished by a dual blood supply: blood vessels within the inner retinal layers and the choroid.
The vasculature of the inner retina is located far enough anteriorly to the photoreceptors that light is able to navigate around it.1 This system is made up of deep and superficial capillary beds that are responsible for nourishing the inner two thirds of the retina. By contrast, the outer one third of the retina is supplied by the choroidal vasculature.1 These two distinct blood supplies are separated by the retinal pigmented epithelium.
Because of this dual supply, neovascularization in proliferative disease processes can arise either from the primary inner vasculature— termed retinal neovascularization—or the vasculature in the choroid, called choroidal (or subretinal) neovascularization. In either case, these new vessels invade areas where vessels are not normally present.2
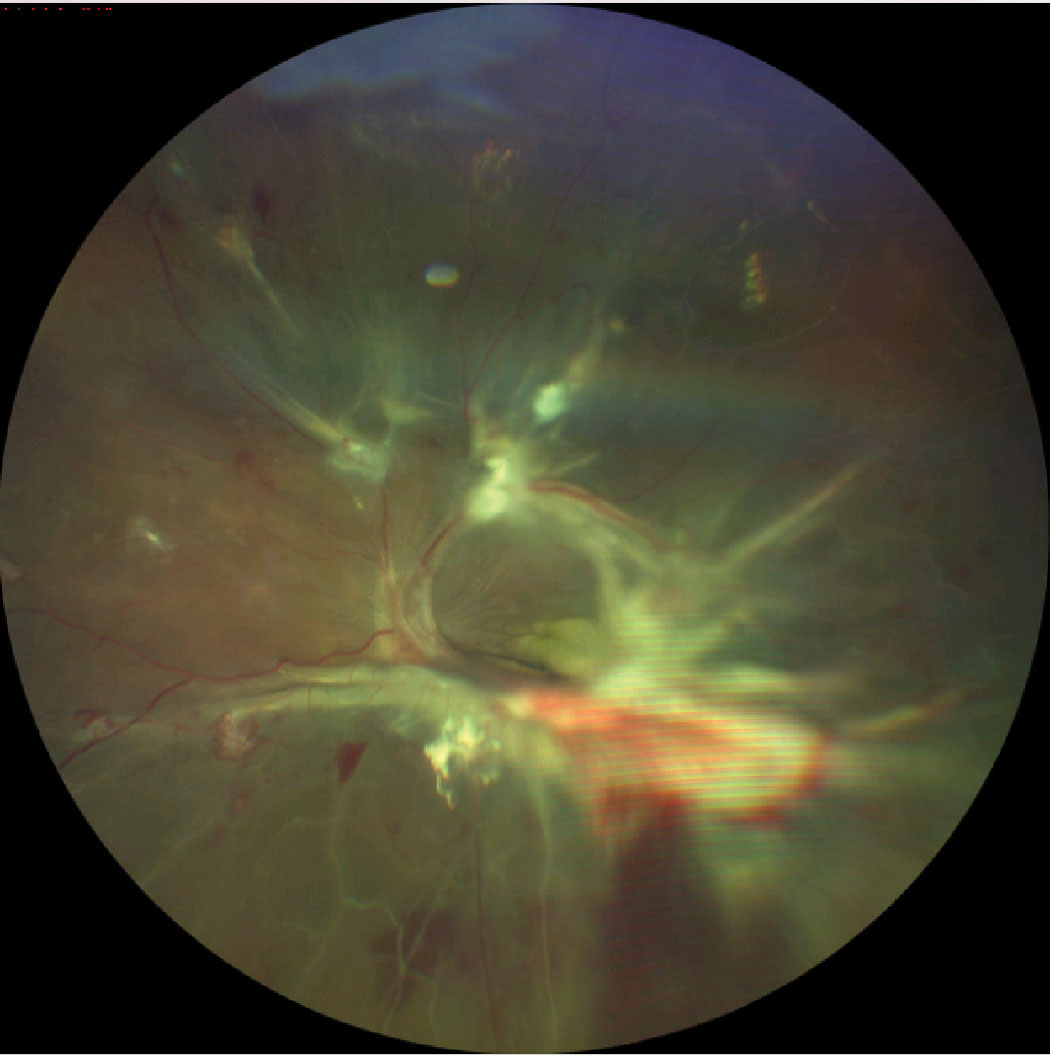 |
| Severe neovascularization with hemorrhage and fibrous proliferation in diabetic retinopathy, leading to tractional retinal detachment. Click image to enlarge. |
Protective Measures
Neovascularization is a protective mechanism that many tissues throughout the body have in response to injury. For example, wound repair in the skin involves the formation of new blood vessels to compensate for those that have been damaged.1 In the retina, disease processes that cause damage to normal retinal vasculature, leading to ischemia and retinal nonperfusion, typically stimulate the growth of neovascularization.
A key contributor that has been heavily studied in this process is vascular endothelial growth factor (VEGF). While normally present in healthy eyes, VEGF is highly expressed in proliferative disease, triggering the growth of neovascularization. When photoreceptors and neurons are deprived of oxygen and nutrients that are usually supplied by healthy vessels, the resultant hypoxia triggers VEGF release.3 VEGF is known to stimulate and mediate vasculogenesis, endothelial cell migration and tube formation.4
Retinal Neovascularization
Although these new blood vessels are formed to compensate for a lack of oxygen and nutrients, instead of repairing the problem they exacerbate it. This is attributed to the differences in the structure of neovascularization as compared with normal, healthy retinal vasculature.
Unlike regular retinal vessels, neovascularization is composed of thin-caliber vessels that lack tight junctions.1,2 One may recall that tight junctions of the retinal vasculature are a key feature, comprising one of the blood/retinal barriers.1 As such, these new blood vessels are very prone to leakage and exude plasma into the nearby tissue and vitreous. This causes the vitreous gel to degenerate, contract and collapse, ultimately leading to devastating visual complications such as vitreous hemorrhages and tractional retinal detachment.1,4 Common etiologies that give rise to proliferative retinal disease include diabetic retinopathy, retinopathy of prematurity, retinal vein occlusion, sickle cell or other hemoglobinopathies and Eales disease.1,2,5
To identify retinal neovascularization, key features on fundus exam help distinguish it from normal retinal vasculature. Besides the smaller and thinner caliber—appearing as fine tufts or fronds—retinal neovascularization is often accompanied by connective or fibrotic tissues that increase in intensity over time. It may appear near the disc (NVD) or elsewhere (NVE), growing either superficially toward the vitreous or down beneath the retina.2
Because of the architecture of retinal neovascularization, fluorescein angiography (FA) shows leakage of dye from these vessels into the extravascular space. Another distinct feature on FA: the neovascular vessels are often located adjacent to areas of poor capillary perfusion to compensate for this pathology.2,6,7
A newer noninvasive method in the works to identify NVE and NVD is OCT angiography (OCT-A). Studies suggest en face OCT-A may visualize these abnormal growths as exuberant vascular proliferation or intense growth of small blood vessels located at the margin of new blood vessels, indicating active proliferation.6
Subretinal Neovascularization
The other piece of the dual blood supply, the outer choroid, is also a site of neovascularization. This is known as subretinal neovascularization, which suggests the new blood vessels grow beneath the retina in the subretinal sensory space.
Subretinal neovascularization can be further subdivided into two categories, depending on the origin of these vessels. The first is retinal angiomatous proliferation, which arises from the deep capillary plexus of the inner retinal vasculature before making its way through the outer retina and into the subretinal space. Choroidal neovascularization, on the other hand, arises from the actual choroidal blood vessels, penetrating Bruch’s membrane and ending in the subretinal space. Regardless of subtype or origin, both forms of subretinal neovascularization are complications of wet (exudative) age-related macular degeneration, among other, less common diseases.
On retinal examination, subretinal neovascularization appears as a greenish or grayish lesion, which may or may not be associated with retinal hemorrhages, exudate or edema. Leakage is also present on FA. Using OCT-A, subretinal neovascularization can be seen as a “seafan” or vascular complex within the outer retina, which is otherwise devoid of blood vessels. These complexes are often located in areas where there is less than optimal perfusion.8
Treatment
Since we understand how and why neovascularization arises, we are able to identify primary therapeutic targets. The mainstay of treatment for several decades has been the use of panretinal photocoagulation. This laser therapy aims to destroy areas in the peripheral retina in the hopes of diminishing VEGF release, thereby regressing neovascularization. Though effective, it is not without side effects, such as reduced peripheral vision, loss of night vision, pain, blur and macular edema.7
Anti-VEGF agents are also available through intravitreal injection, again attempting to retard the stimulus of neovascularization. Currently available anti-VEGF drugs include pegaptanib, bevacizumab, ranibizumab, aflibercept, brolucizumab and faricimab, along with some biosimilars.
As we continue to research stimuli for neovascular formation, therapeutic targets are more easily identifiable. However, one thing is for certain: identifying these conditions quickly and accurately is critical in ensuring the best treatment outcome.
Dr. Labib graduated from Pennsylvania College of Optometry, where she now works as an associate professor. She completed her residency in primary care/ocular disease and is a fellow of the American Academy of Optometry and a diplomate in the Comprehensive Eye Care section. She has no financial interests to disclose.
1. Campochiaro PA. Ocular neovascularization. J Mol Med. 2013;91(3):311-21. 2. Henkind P, Wise GN. Retinal neovascularization, collaterals, and vascular shunts. Br J Ophthalmol. 1974;58(4):413-22. 3. Fu Z, Sun Y, Cakir B, et al. Targeting neurovascular interaction in retinal disorders. Int J Mol Sci. 2020;21(4):1503. 4. Takagi H. Molecular mechanisms of retinal neovascularization in diabetic retinopathy. Intern Med. 2003;42(3):299-301. 5. Cabral T, Mello LGM, Lima LH, et al. Retinal and choroidal angiogenesis: a review of new targets. Int J Retina Vitreous. 2017;3:31. 6. Ishibazawa A, Nagaoka T, Yokota H, et al. Characteristics of retinal neovascularization in proliferative diabetic retinopathy imaged by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(14):6247-55. 7. Feng HE, Weihong YU, Dong F. Observation of retinal neovascularization using optical coherence tomography angiography after panretinal photocoagulation for proliferative diabetic retinopathy. BMC Ophthalmol. 2021;21(1):252. 8. Mohla A, Khan K, Kasilian M, Michaelides M. OCT angiography in the management of choroidal neovascular membrane secondary to Sorsby fundus dystrophy. BMJ Case Rep. 2016;2016:bcr2016216453. |

