There are a number of clinical conditions associated with the peripheral retina, including primary lesions such as pars plana cysts. Some are degenerative, with potential vision-threatening consequences. Others are associated with systemic disease and may better correlate with the patients’ presenting signs and symptoms.
 |
| Click image to enlarge. |
Dilated fundus examination remains the standard of care for detection and evaluation of these findings. However, advances in imaging technologies, such as widefield and ultra-widefield photography and optical coherence tomography (OCT), have given us new, valuable tools in the differential diagnosis and management of not only central, but also peripheral, retinal pathology.
This review discusses various lesions, their morphology and their long-term prognosis and includes a pictorial review of those that I’ve seen throughout my years of experience with this particular area of expertise.
Explore the Landscape
 |
| Click image to enlarge. |
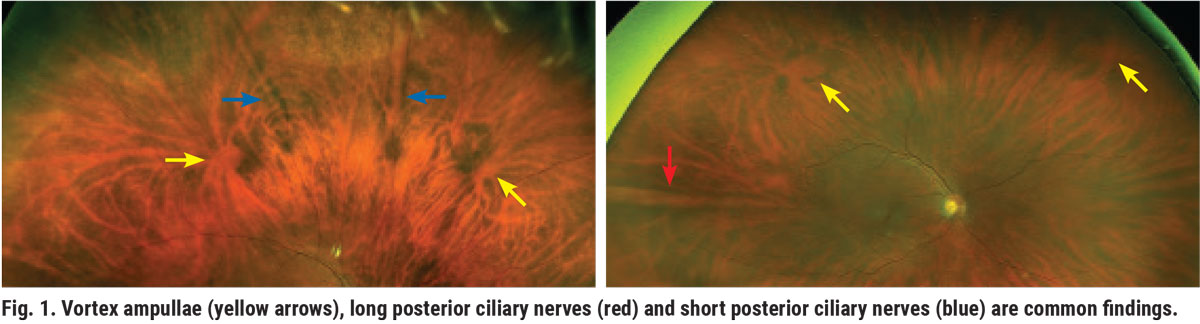
There are certain anatomical features we need to first familiarize ourselves with as we extend the fundus examination from the posterior pole to the peripheral retina. The vortex ampullae located in the equatorial retina are one of them (Figure 1). Vortex veins have a variety of shapes and sizes.
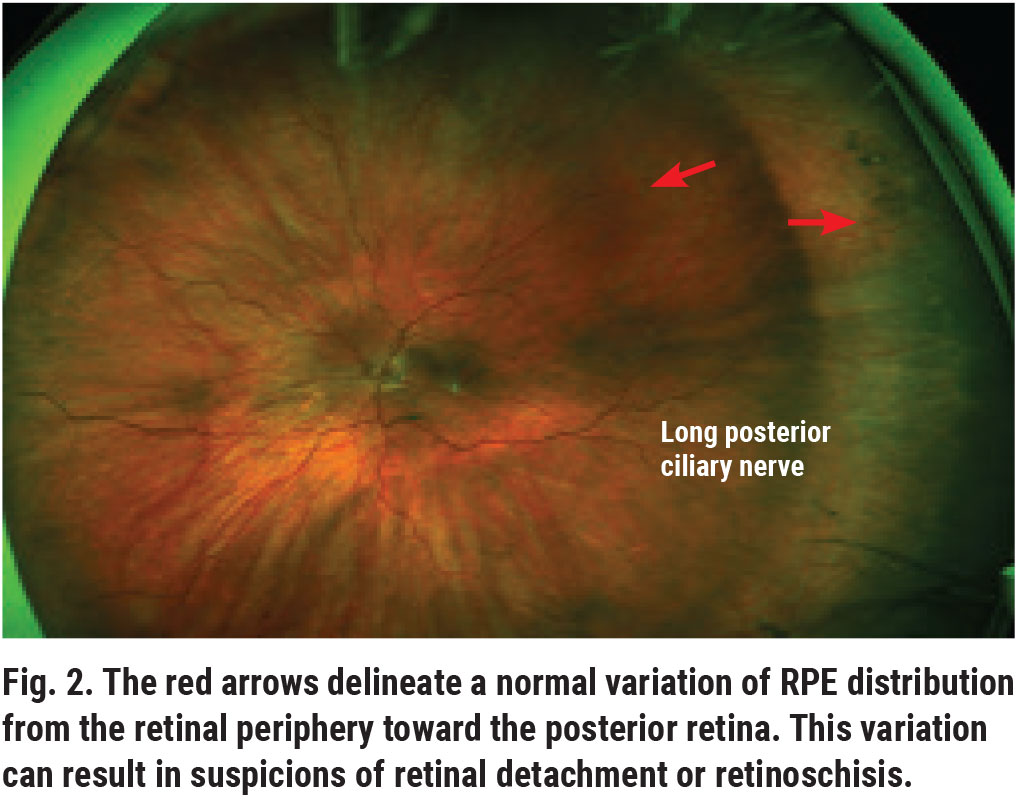
The region anterior to the equator is the peripheral retina. Distribution differences in the retinal pigment epithelium (RPE) between the peripheral and central retina can result in unusual appearances and cause diagnostic dilemmas (Figure 2).
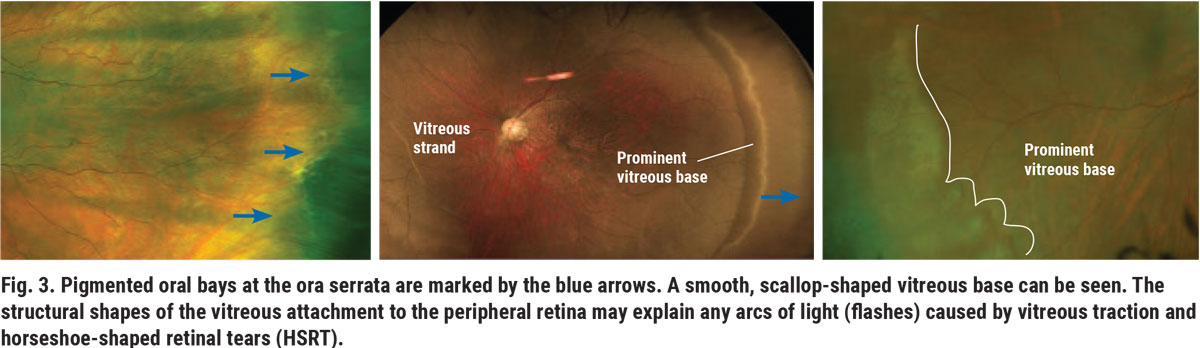
Other important anatomical landmarks include the ora serrata, the serrated region between the retina and ciliary body (Figure 3). Ora serrations, or oral bays, have varying degrees of pigmentation, shapes and sizes, often resulting in diagnostic challenges.
 |
| Click image to enlarge. |
The vitreous base, a band of vitreous attachment extending 2mm anterior and 1mm to 3mm posterior to the ora, is more prominent and visible depending on the retinal sector and patient (Figure 3).
Another common normal retinal finding is the spear-shaped long and short posterior ciliary nerves (Figure 1). The long nerves are more prominent and are usually located in sectors three and nine. The short ciliary nerves can be seen at times in the sectors between three and nine.
 |
| Click image to enlarge. |
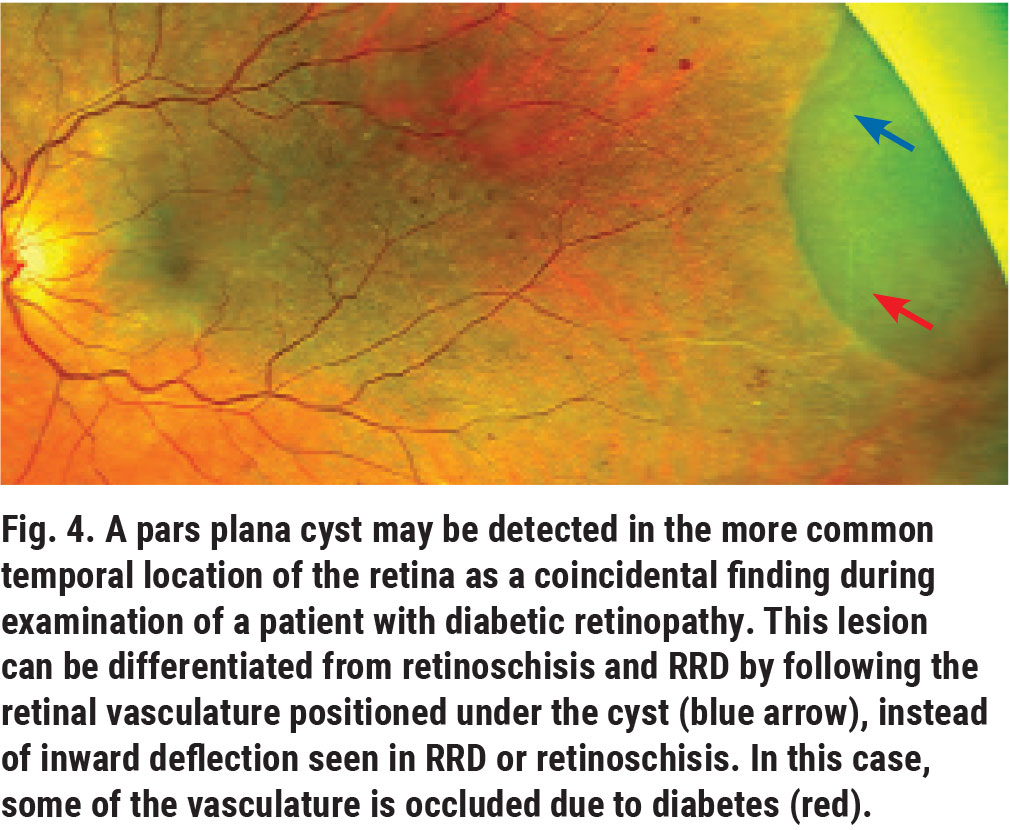
Pars plana cysts are clear, bullous balloon-like structures that extend from the non-pigmented ciliary epithelium of the pars plana to the peripheral retina under the vitreous cortex (Figure 4). They more commonly appear in the temporal periphery and affect 5% to 10% of the global population.1 Due to their location and the clear fluid (hyaluronic acid) they are usually filled with, they can go undetected.
These benign lesions can resemble small rhegmatogenous retinal detachments (RRD) or retinoschiss, but, as a distinction, the retinal vessels under the lesion are visible on close examination. The fluid content of these cystic structures can appear cloudy in the presence of abnormal serum protein, such as in patients with multiple myeloma.
 |
| Click image to enlarge. |
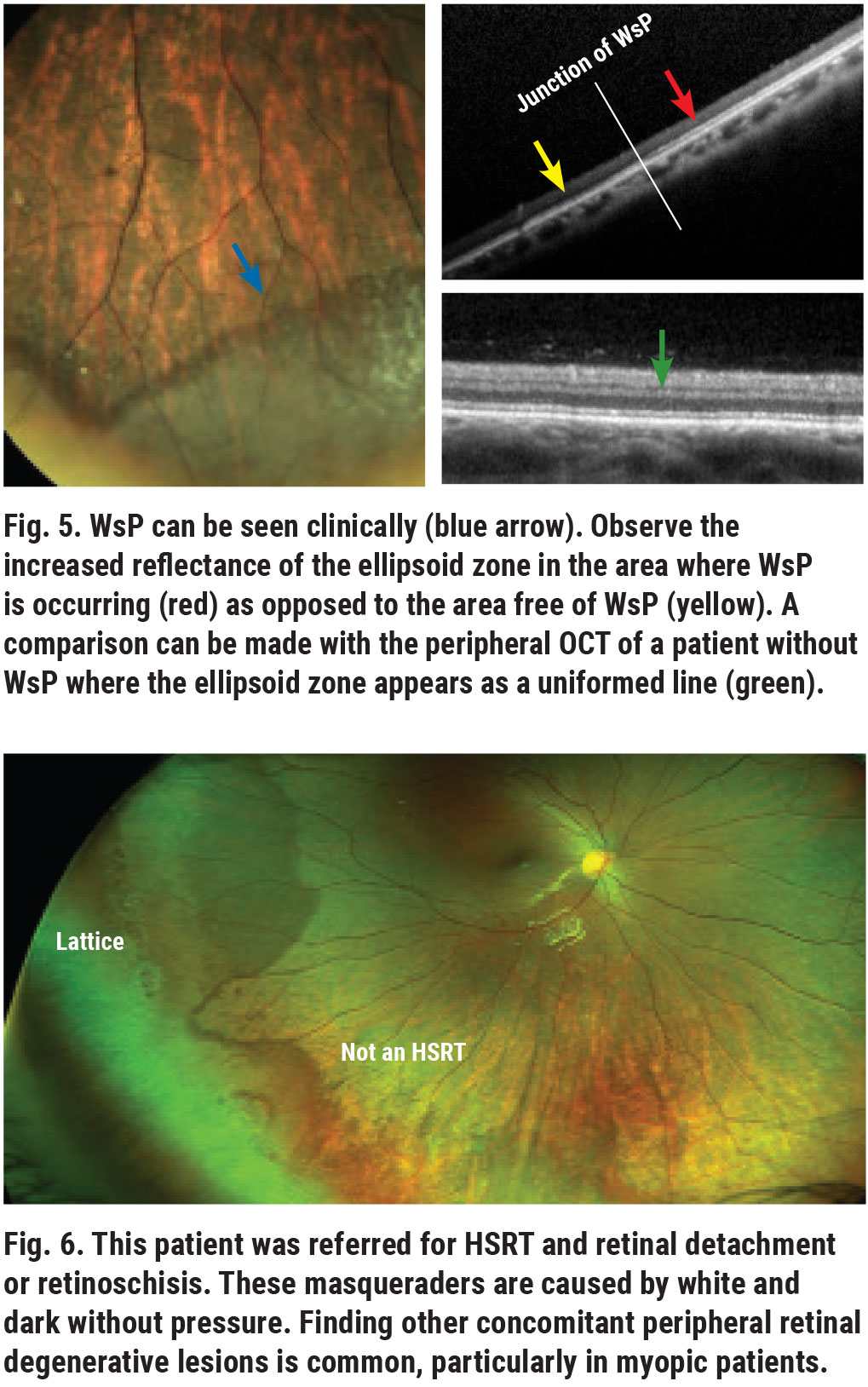
White and dark without pressure are also common peripheral retinal findings. White without pressure (WsP) geographic areas appear as “retinal whitening” without scleral depression and are usually located in the equatorial and peripheral retina.2 Traditional thinking attributes the etiology of this condition to the vitreoretinal interface. However, this line of thought was disputed by examination with spectral-domain OCT, which shows thickening and increased hyperreflectivity of the ellipsoid zone, formally known as the IS-OS line (Figure 5).3
WsP is usually a coincidental, inconsequential finding, but it can result in diagnostic dilemmas in the differentiation of retinal breaks, RRD and retinoschisis (Figure 6). Also, as WsP is more prevalent in high myopia, these patients have a higher incidence of other peripheral retinal degenerative disease and RRD.
Dark without pressure regions, on the other hand, appear as darker areas compared with the surrounding retina. These usually do not increase the risk of RRD but are often seen in eyes that also have WsP and other peripheral retina degenerative findings. OCT imaging of dark without pressure shows findings opposite those of WsP; the ellipsoid zone appears less reflective than the adjacent area (Figure 7).
 |
| Click image to enlarge. |
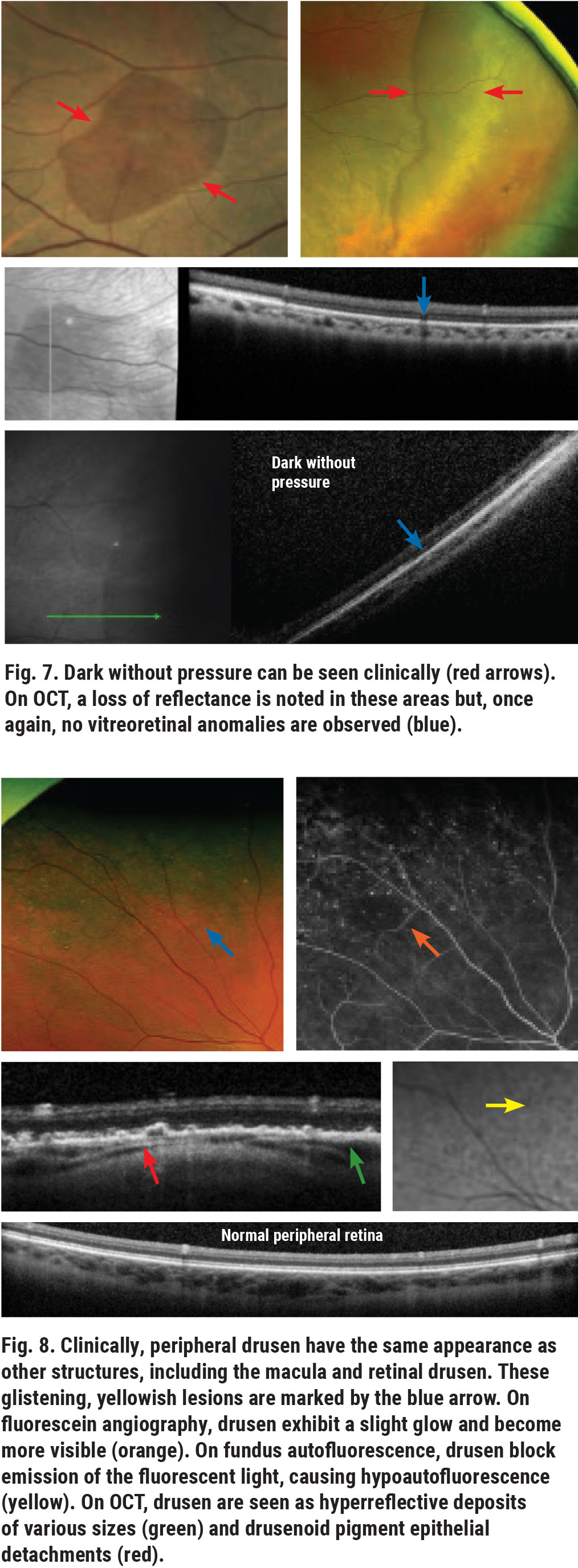
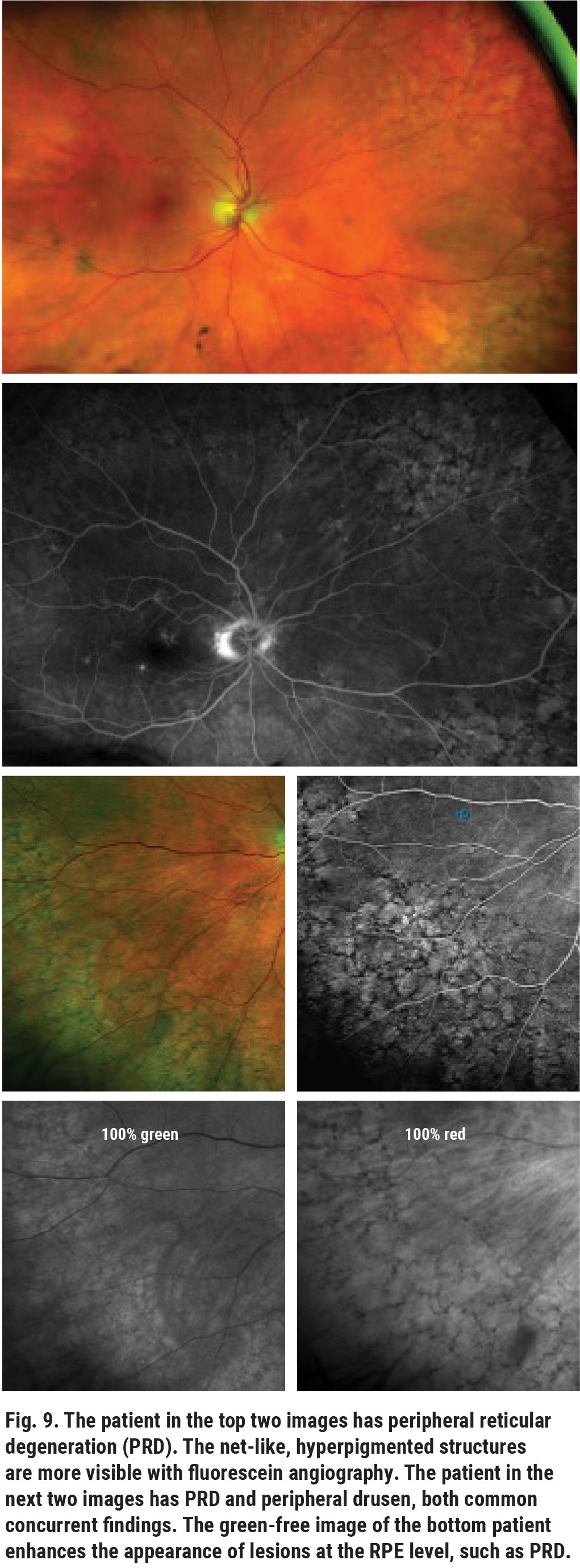
Peripheral drusen and peripheral reticular degeneration (PRD) are common and often underreported peripheral retinal findings that can be seen in patients with or without macular degeneration. Although these are both related to age, they are two distinct conditions with two different phenotypes and should be treated as such. As they are alterations of the outer retina, they do not pose a risk for RRD. However, the phenotypic (clinical, OCT and angiographic) findings of peripheral degeneration are similar to those of macular drusen (Figure 8). Reticular pigmentation appears as a net-like pigmented structure that may look similar to the pigmentary abnormalities associated with macular degeneration (Figure 9).
 |
| CAPTION Click image to enlarge. |
There are also genotypic associations between peripheral degeneration findings and age-related macular degeneration (AMD).4 Additionally, peripheral drusen have also been considered a potential marker for Alzheimer’s dementia (AD). Peripheral drusen and degeneration should not be used as diagnostic indicators for AD or AMD but should encourage practitioners to more thoroughly monitor patients with these two peripheral retinal degenerative conditions.5
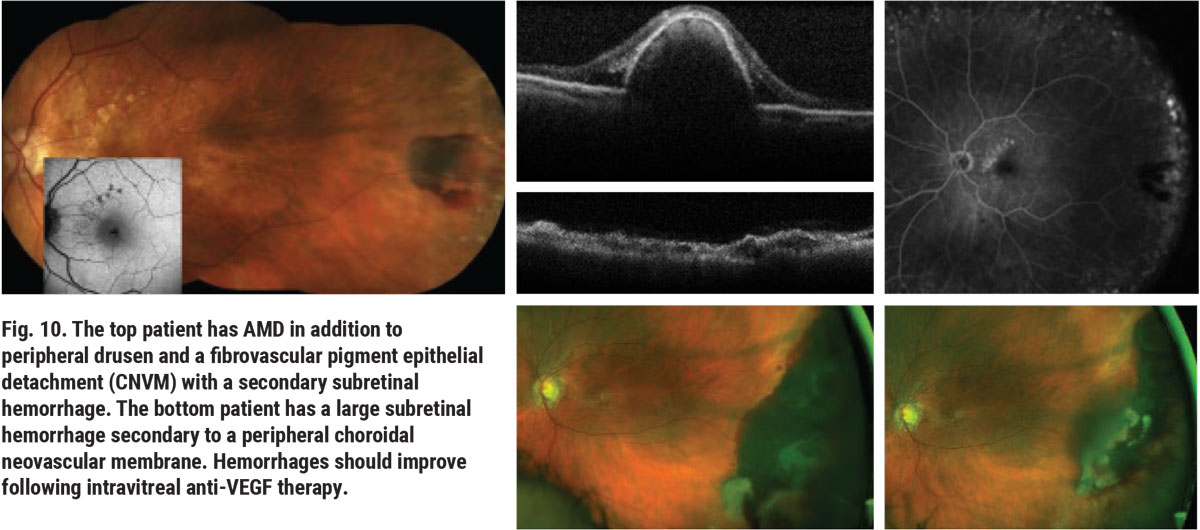
Choroidal ischemia has also been associated with PRD.6 Peripheral choroidal neovascular membranes (CNVMs) can appear in cases of peripheral drusen as coincidental findings or in patients with associated symptoms caused by preretinal hemorrhage, vitreous hemorrhage or both (Figure 10). Peripheral CNVMs can be effectively treated with anti-VEGF injections in the same fashion as macular CNVMs, though they are often monitored without need for treatment. Patients with associated vitreous hemorrhage may require pars plana vitrectomy.
 |
| Click image to enlarge. |
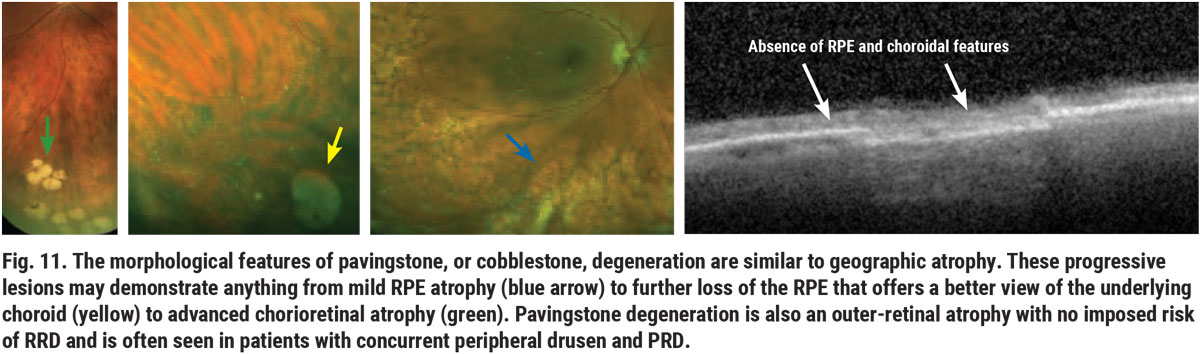
Pavingstone (or cobblestone) degeneration is another outer-retinal and chorioretinal degeneration that does not impose a risk for rhegmatogenous retinal detachment. Pavingstone degeneration may share similar findings with geographic atrophy (Figure 11). These lesions, as well as PRD and peripheral drusen, are associated with choroidal vascular and possibly carotid artery insufficiency.6 This is not to suggest that every patient with these findings requires a carotid artery disease (CAD) workup; however, if other comorbidities are present, such as smoking and lipid disorder, evaluation for carotid artery disease may be warranted. Choroidal thinning is also an associated feature of AMD and myopic macular degeneration.7
 |
| Click image to enlarge. |
 |
| Click image to enlarge. |
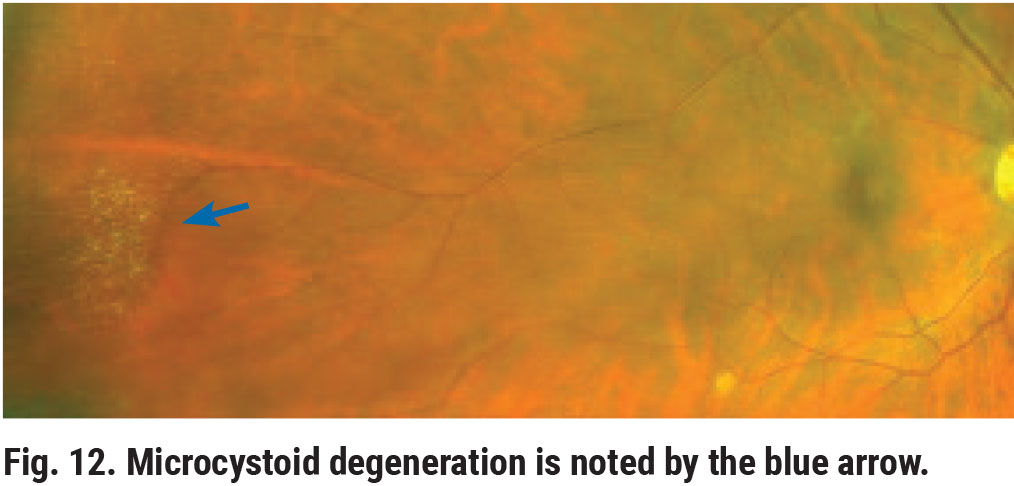
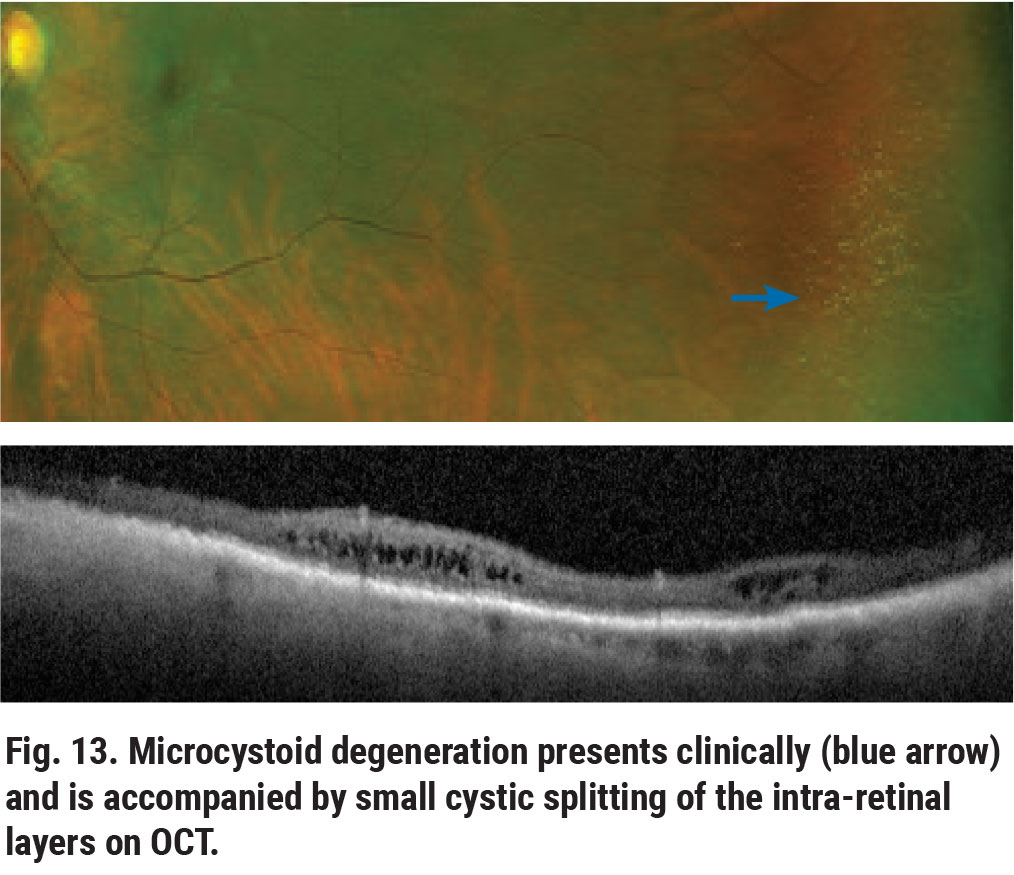
Peripheral microcystoid degeneration is the most common intra-retinal degeneration.8,9 Clinically, microcystoid degeneration presents as small, yellowish bubble-like aggregates in the retinal periphery (Figure 12). On OCT, it appears as small cystic spaces involving the inner nuclear and outer plexiform layers (Figure 13). In itself, microcystoid degeneration is a benign condition; however, it is often a precursor to peripheral acquired retinoschisis.8
 |
| Click image to enlarge. |
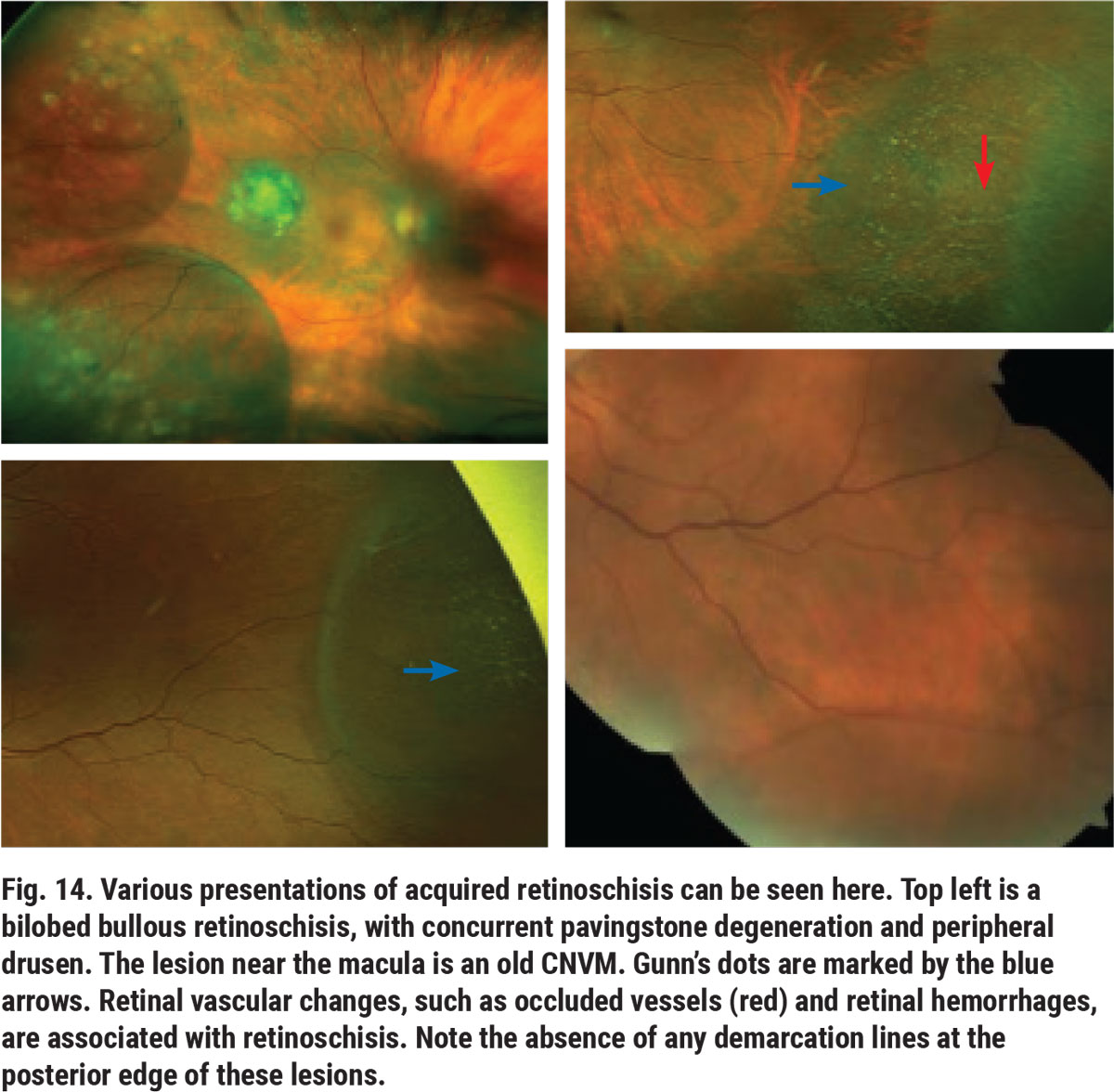
Peripheral acquired or age-related retinoschisis is a splitting in the neurosensory retina. This idiopathic and often progressive condition can result in bullous or balloon-like areas in the temporal or inferotemporal retinal periphery (Figure 14). On clinical examination, these lesions can have a wide range of widths and heights. They usually have small glistening or refractile deposits known as Gunn’s dots, which are seen in the inner area of the schisis cavity (Figure 14).
 |
| Click image to enlarge. |
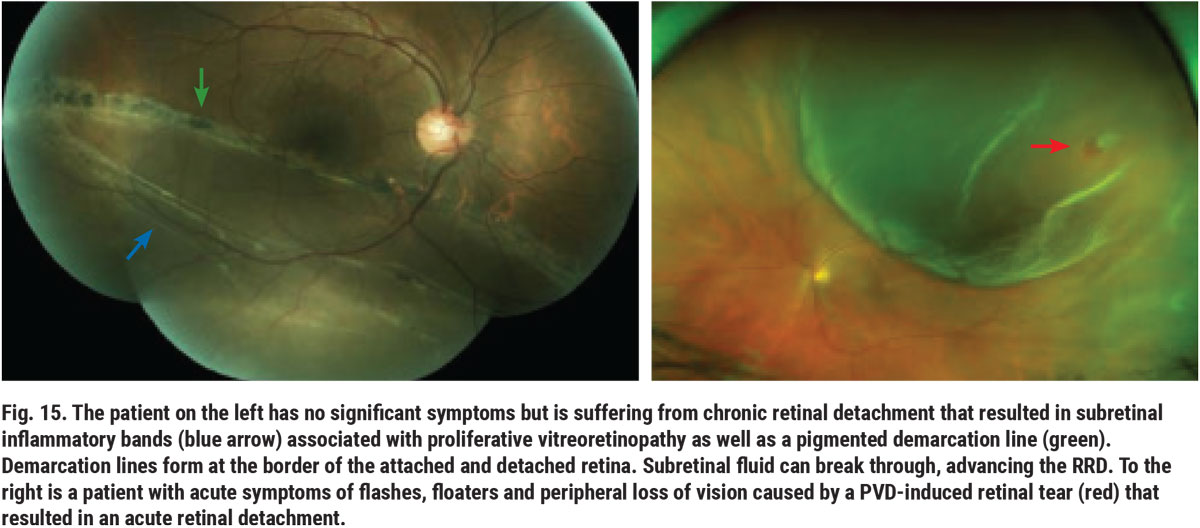
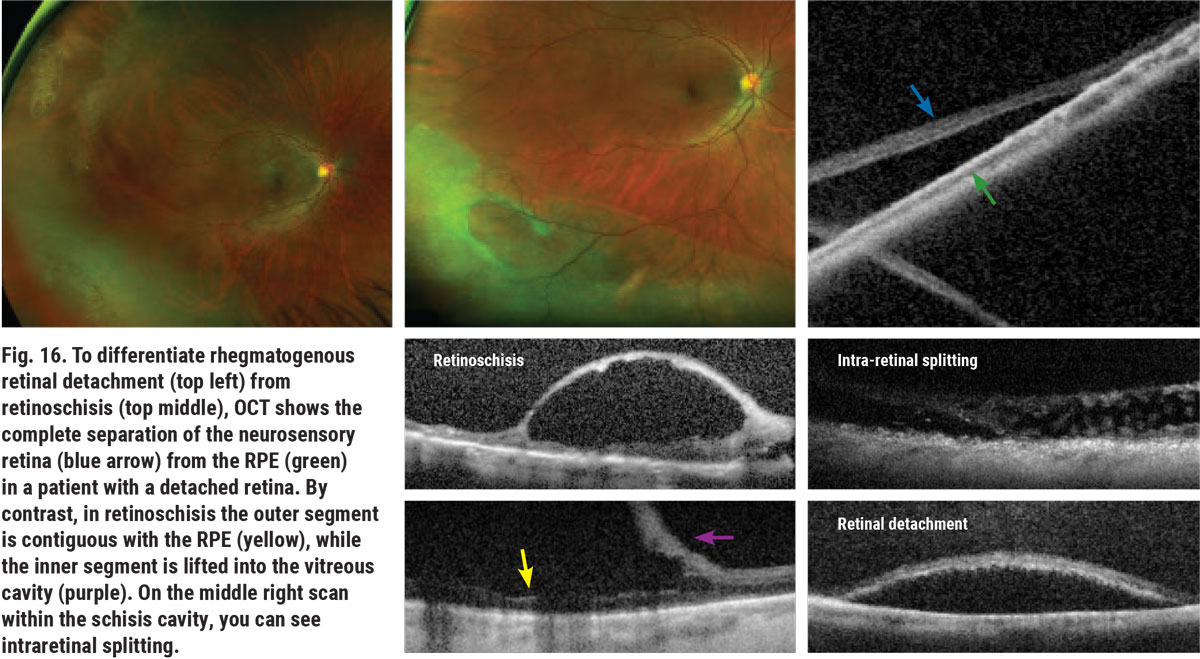
Differentiating retinoschisis from RRD can be a challenging task.10 Shallow retinoschisis can resemble a chronic RRD. Distinguishing factors between the two include the absence of a demarcation line that is associated with RRD, as well as the absence of a full-thickness retinal hole or break. Bullous retinoschisis can appear as acute RRD. A patient’s symptomatology and the presence or absence of retinal breaks helps aid in telling the two apart (Figure 15). Patients with RRD often are or eventually become symptomatic; whereas, in most cases, retinoschisis is a coincidental finding. OCT can assist in differentiating between the two (Figure 16). Slit lamp assessment may also be a helpful option, as the tool can determine whether the patient is able to see the light. Patients with retinoschisis will also have an absolute scotoma in the affected area, obstructing their vision.
 |
| Click image to enlarge. |
 |
| Click image to enlarge. |
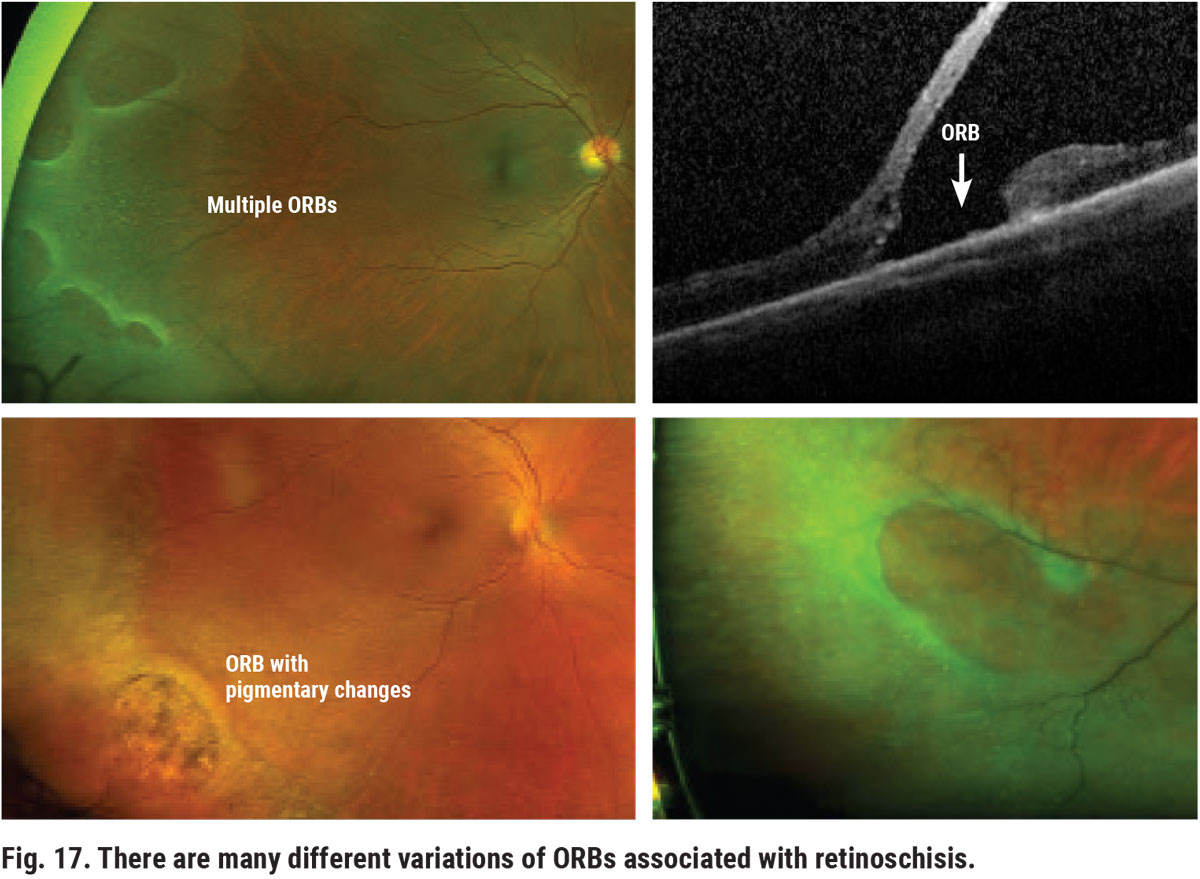
The relative risk that retinoschisis poses increases as certain degenerative changes occur. Atrophic changes over time can result in loss of the outer retina in at least one location. These areas are called outer-retinal breaks (ORBs) within the schisis cavity and may have various appearances, including altered pigmentation and round or oval red or orange holes (Figure 17). As long as the inner layers of the schisis remain intact, the fluid flux between the vitreous side and RPE remains normal with no increased risk of RRD. However, if a hole or tear develops on the inner-side of the schisis in the presence of ORBs, the patient will become more prone to RRD (Figure 18).
 |
| Click image to enlarge. |
 |
| Click image to enlarge. |
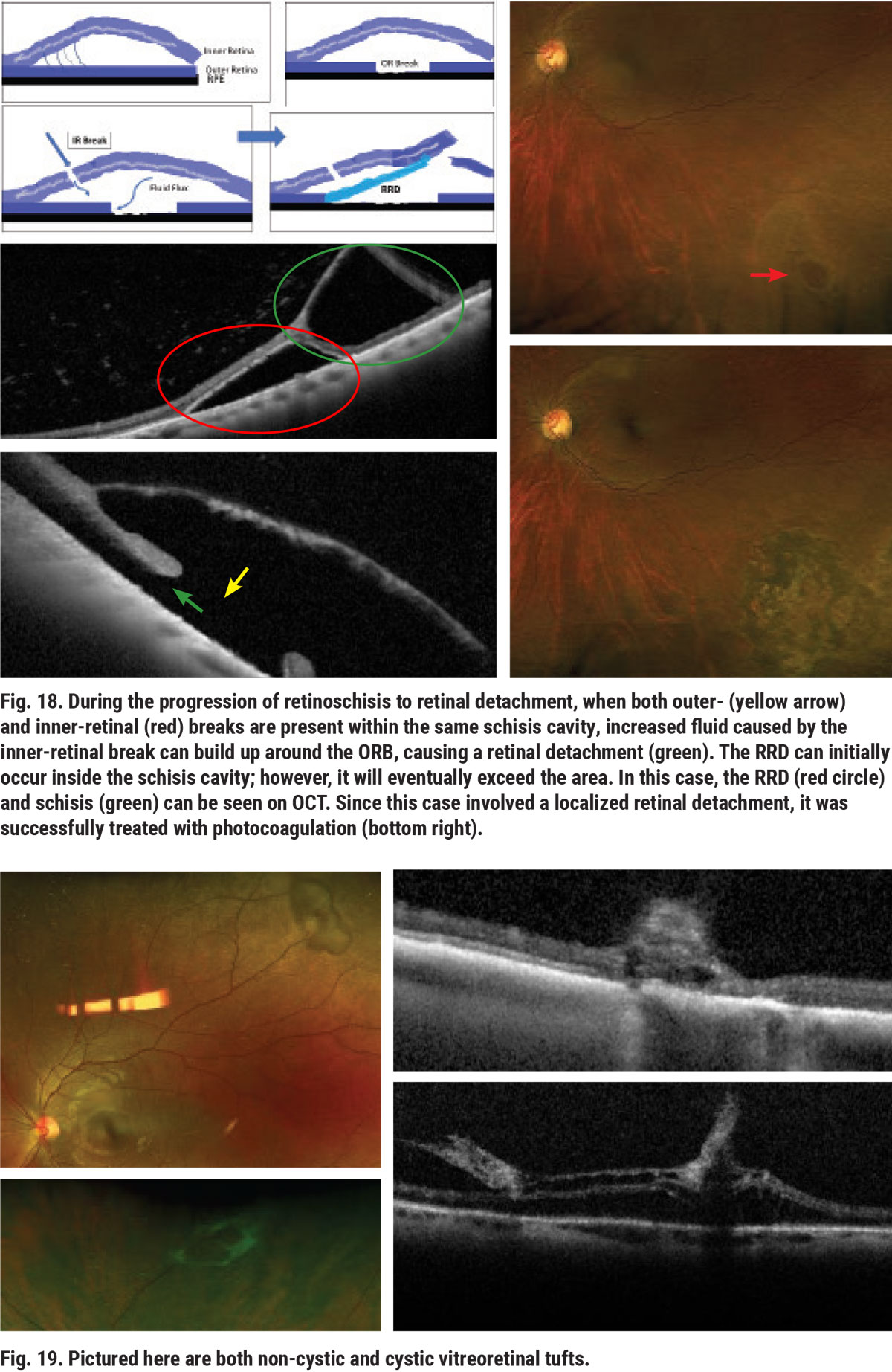
Peripheral vitreous tufts are caused by congenital abnormality of the vitreoretinal interface.11 Tufts can have cystic and non-cystic features (Figure 19). Vitreoretinal tufts have a low risk of leading to RRD. A vitreous tuft with a higher tensile strength than the retina at the site of its attachment may cause a retinal tear during posterior vitreous detachment (PVD) and serve as a precursor to RD (Figure 20).11,12
 |
| Click image to enlarge. |
 |
| Click image to enlarge. |
 |
| Click image to enlarge. |
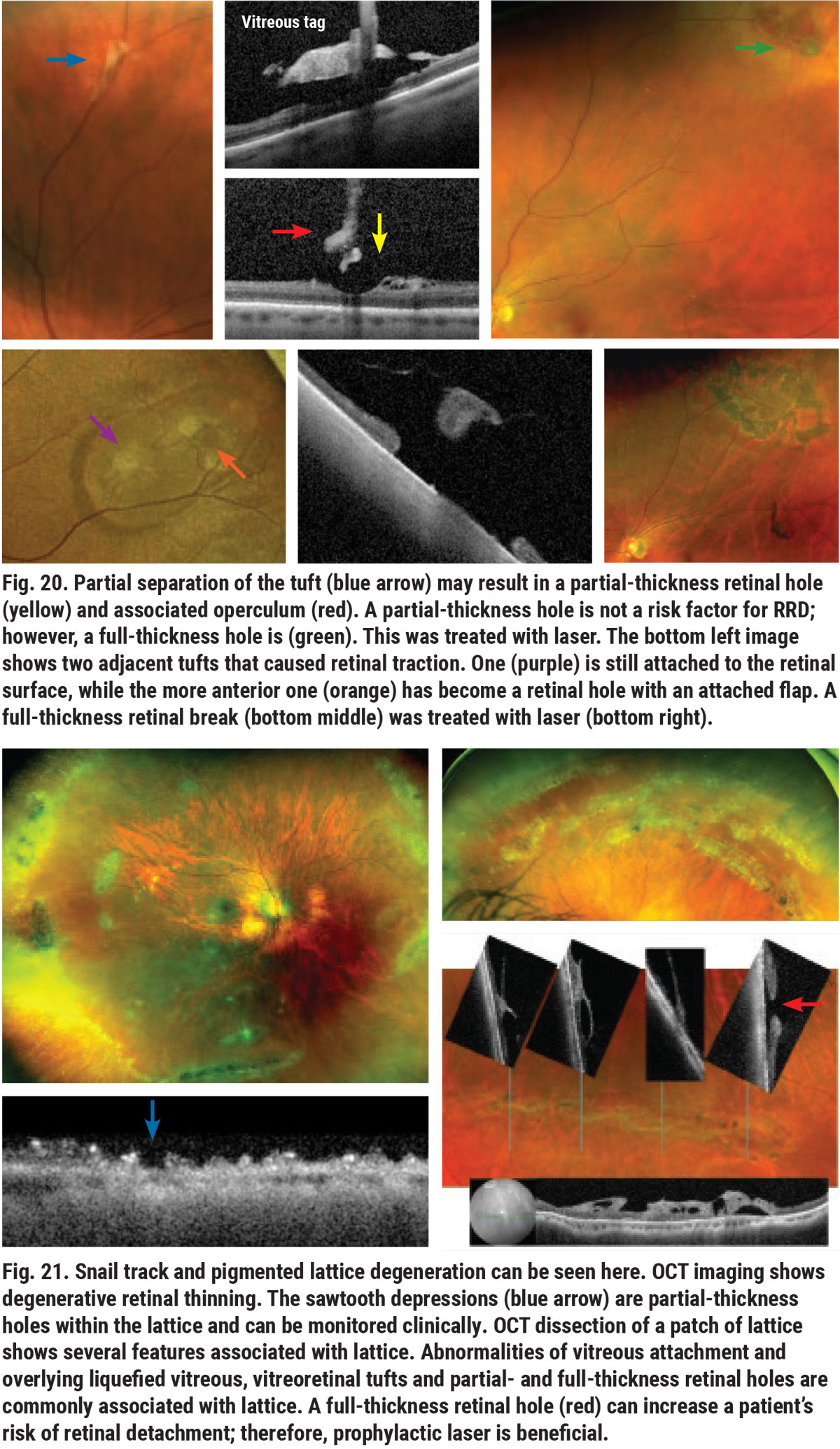
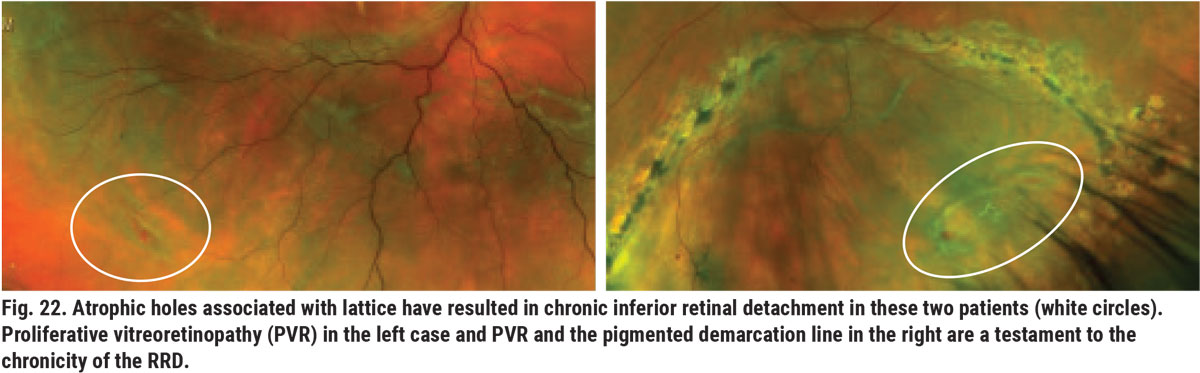
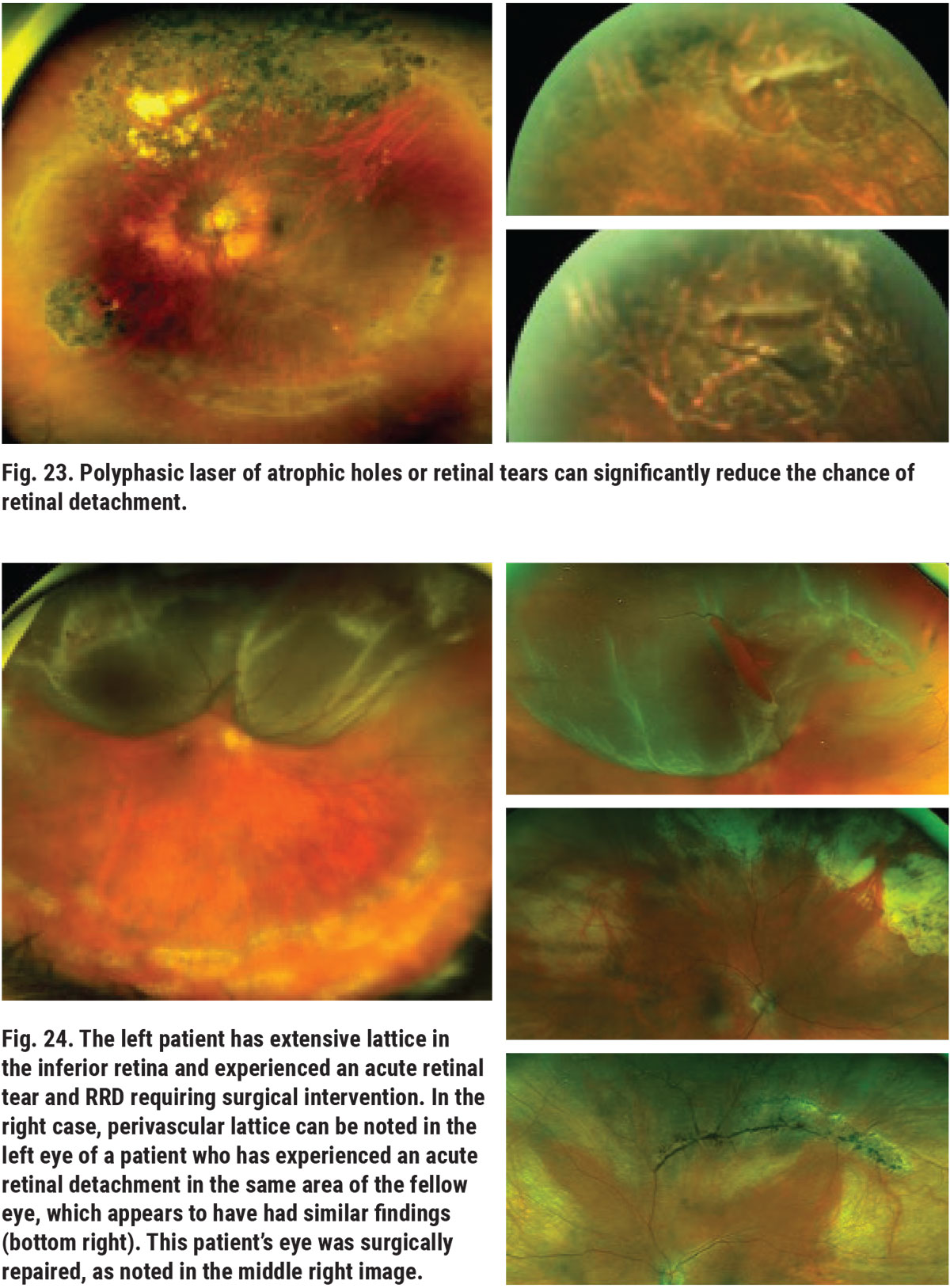
Lattice degeneration is a common peripheral retinal (or vitreoretinal) degeneration that results in abnormal thinning of peripheral retina. There are various clinical appearances of lattice; some have white lines, referred to as snail track, and some are pigmented. Partial- and full-thickness retinal holes and breaks are often found within and/or adjacent to the patches of lattice (Figure 21). Full-thickness retinal holes can predispose the patient to the development of slowly progressing RRD (Figure 22). Prophylactic laser around full-thickness atrophic holes can reduce this threat (Figure 23). During the process of PVD, areas affected by lattice degeneration are more easily torn, leading to acute RRD, which requires surgical intervention (Figure 24).13
Conclusion
Peripheral retinal degenerative conditions are common, and often seen concomitantly. Predisposing factors include age and myopic refractive error; however, these may also occur as coincidental findings. Since vision-threatening sequelae serve as potential side effects, dilated fundus examination and ancillary imaging are crucial to determine the need for prevention, treatment and proper follow-up.
Dr. Rafieetary is a consultative optometric physician at the Charles Retina Institute in Germantown, TN. He is on advisory boards for Heidelberg Engineering, Optos, Regeneron, Notal Vision and Cardinal Health.
1. Braceros KK, Choi D, Gallemore R. Case report on giant pars plana cysts mimicking retinal detachment. Int Med Case Rep J. 2020;13:191-4. 2. Wilkinson C, Hinton D, Ryan S, eds. Retina. Philadelphia: Elsevier; 2006. 3. Diaz RI, Sigler EJ, Randolph JC, et al. Spectral domain optical coherence tomography characteristics of white-without-pressure. Retina. 2014;34(5):1020-1. 4. Seddon JM, Reynolds R, Rosner B. Peripheral retinal drusen and reticular pigment: association with CFHY402H and CFHrs1410996 genotype in family and twin studies. Invest Ophthalmol Vis Sci. 2009;50(2):586-91. 5. Ritchie CW, Peto T, Barzegar-Befroei N, et al. Peripheral retinal drusen as a potential surrogate maker for Alzheimer’s dementia: a pilot study using ultra-wide angle imaging. Invest Ophthalmol Vis Sci. 2011;52:6683. 6. Bae K, Cho K, Kang SW, et al. Peripheral reticular pigmentary degeneration and choroidal vascular insufficiency, studied by ultra wide-field fluorescein angiography. PLoS One. 2017;12(1):e0170526. 7. Sigler EJ, Randolph JC. Comparison of macular choroidal thickness among patients older than age 65 with atrophic age-related macular degeneration and normals. Invest Ophthalmol Vis Sci. 2013;54:6307-13. 8. Hines JL, Jones WL. Peripheral microcystoid retinal degeneration and retinoschisis. J Am Optom Assoc. 1982;53(7):541-5. 9. Chhablani J, Bagdi AB. Peripheral retinal degenerations. American Academy of Ophthalmology. eyewiki.aao.org/Peripheral_Retinal_Degenerations. Updated October 1, 2020. Accessed January 19, 2021. 10. Rafieetary M, Huddleston S, Attar R. Rhegmatogenous retinal detachment: how to detect, how to manage. Rev Optom. 2017;154(9):78-84. 11. Rafieetary M, Huddleston S. A field guide to retina holes and tears. Rev Optom. 2019;156(6):52-7. 12. Chu RL, Pannullo NA, Adam CR, et al. Morphology of peripheral vitreoretinal interface abnormalities imaged with spectral domain optical coherence tomography. J Ophthalmol. June 9, 2019. [Epub ahead of print]. 13. Kim TI. Lattice degeneration. American Academy of Ophthalmology. eyewiki.aao.org/Lattice_Degeneration. Updated December 18, 2020. Accessed January 19, 2021. |

