| About this Series To help optometrists strengthen their protocols for managing conditions that require ongoing—perhaps life-long—care, this series explains the steps to take after confirming a diagnosis, from day one through long-term management. Each installment in the five-part “Now What?” series will cover a different chronic condition: My Patient Has Glaucoma...Now What? My Patient Has Recurrent Corneal Erosion...Now What? June—scleritis July—AMD |
You have dilated your diabetic patient and their fundus shows signs of retinopathy (Figure 1). Now it’s time to determine your next step. Do you monitor the patient or refer to a retina specialist? The answer can be somewhat tricky because multiple practice guidelines for managing diabetic retinopathy (DR) exist, both from optometry and ophthalmology in the United States, as well as internationally.1-3
Any therapeutic protocol will depend upon staging the severity of the DR. Note the location of any diabetic changes in the fundus. Next, classify the DR as either non-proliferative (NPDR) or proliferative (PDR). NPDR should be labeled as mild, moderate, severe or very severe. PDR can be further categorized into non-high risk and high risk (Table 1).
Finally, with all levels of retinopathy, you should definitively determine the presence or absence of macular edema. So, what are the signs for the various stages of DR that allow practitioners to effectively categorize the disease process?
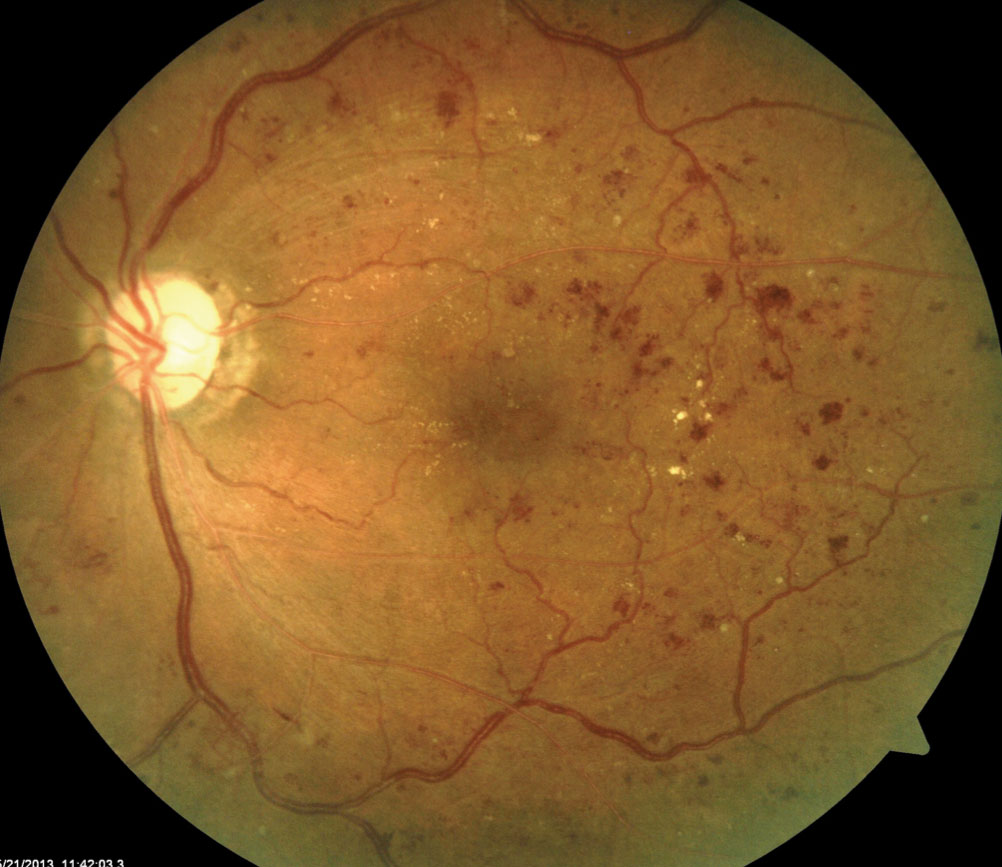 |
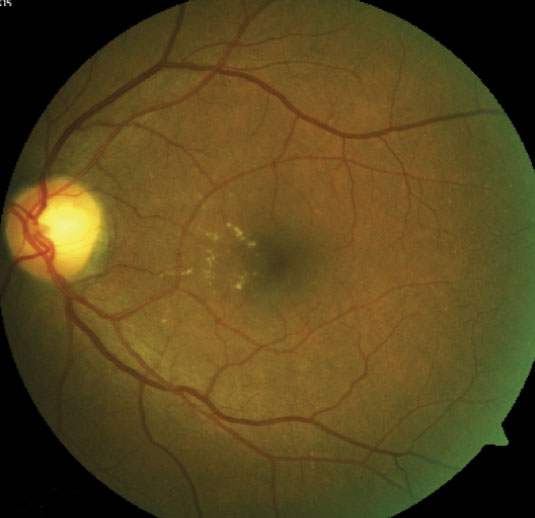
| Fig. 1. This fundus shot shows a patient with signs of moderately severe NPDR. Click to enlarge image. |
Step One: Stage the Severity
In a sense, this is your most important task as it sets the tone for the management decisions to follow.
Mild NPDR. This stage is characterized by microaneurysms, dot/blot hemorrhages or hard exudates or a combination of all three. Further diagnostic testing is not indicated in mild NPDR, but fundus photography is often helpful to establish a baseline level of retinopathy to assess future progression, and is useful for patient education.
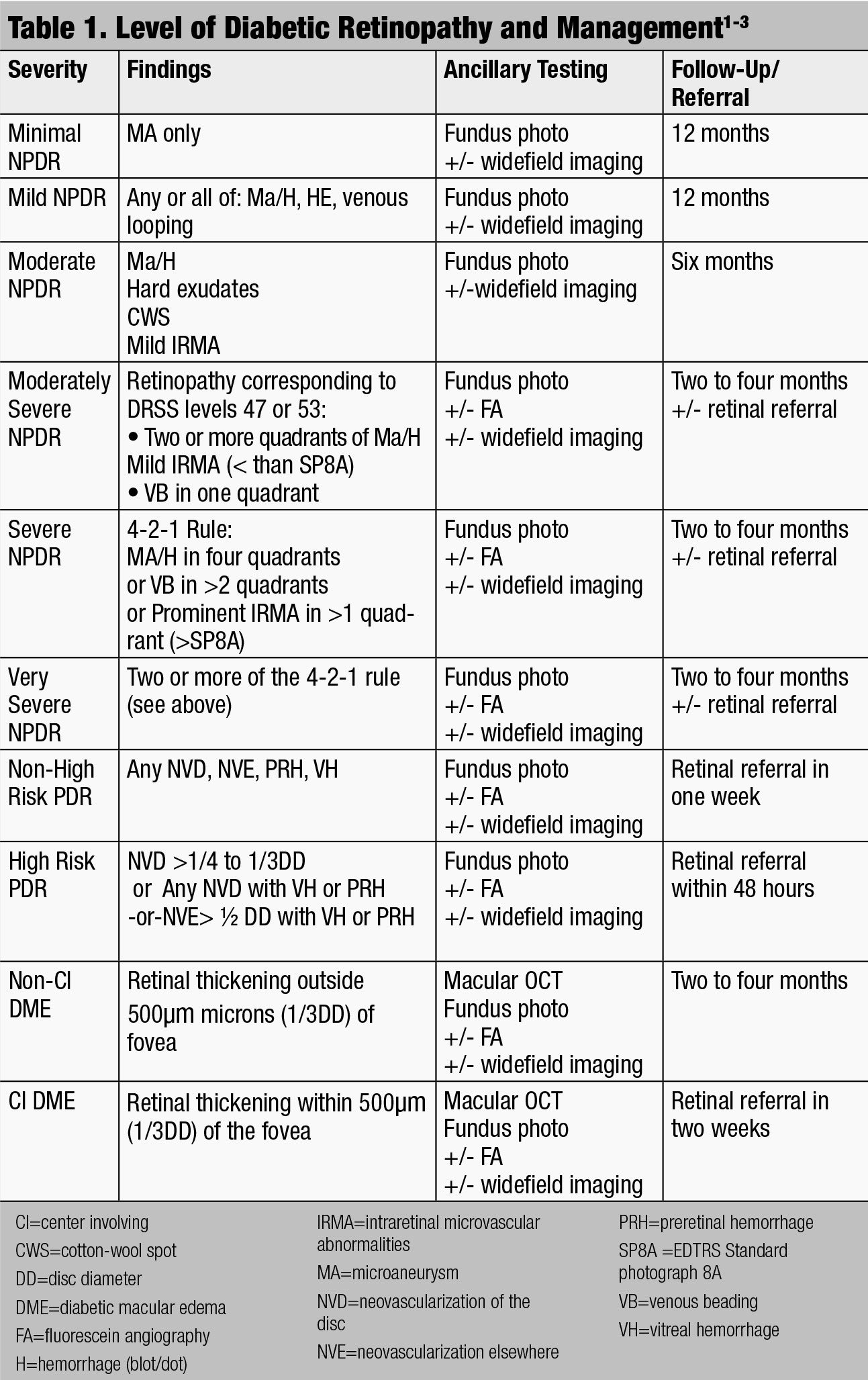
While traditional optical coherence tomography (OCT) is not indicated for mild NPDR unless retinal thickening is observed with stereoscopic clinical examination or visual acuity is reduced, OCT angiography (OCT-A) can be a useful tool to detect capillary dropout and small microvascular changes that are not otherwise noted on dilated fundus exam (Figure 2).
Moderate NPDR. This stage is considered more than just microaneurysms, but less than severe NPDR.2 Moderate NPDR will show an increase in intraretinal hemorrhages and microaneurysms, as well as hard exudates and cotton-wool spots. Mild venous beading (VB) and intraretinal microvascular abnormalities (IRMA) are also seen in moderate NPDR. Fluorescein angiography (FA) may be indicated if VB or IRMA are present. OCT may be appropriate to confirm or rule out the presence of macular edema. Keep in mind that some patients have subclinical diabetic macular edema (DME), only observable with OCT. These patients are at significantly higher risk for worsening DME.4
Moderately severe NPDR. Historically, moderate NPDR was monitored and not treated. However, anti-vascular endothelial growth factor (anti-VEGF) injections are now considered primary therapy to improve DR severity.5-8
Data from anti-VEGF studies such as RISE, RIDE and PANOROMA show patients with moderately severe (or worse) retinopathy have the potential for at least a two-step improvement on the DR Severity Scale (DRSS) when given anti-VEGF drugs such as ranibizumab (Lucentis, Genentech) or aflibercept (Eylea, Regeneron).5-8 Though these patients have not yet developed traditionally treatable disease with either macular edema or proliferative changes, there is value in decreasing the level of retinopathy, potentially delaying or preventing the development of sight-threatening complications.
This is an evolving paradigm, however, and many retina surgeons have not yet embraced this approach. Additionally, it can be difficult to convince patients without sight-threatening changes to undergo repeated intraocular injections.
So, what is the definition of moderately severe NPDR? The definition has not been clearly established in the literature, but many DR trials use the Early Treatment DR Study (ETDRS) severity scale score of 47 or higher.8 This level of DR includes: hemorrhages or microaneurysms, or both, in two or more quadrants, mild IRMA (less than or equal to 0.4mm2) or venous beading in one quadrant.9-11 These findings would be considered severe enough to refer patients for potential anti-VEGF treatment.8 FA may be performed at this stage to better understand the sources of leakage.
Severe NPDR. This stage consists of any one of the following with no signs of proliferative retinopathy: severe intraretinal hemorrhages and microaneurysms in each of four quadrants (> 20 hemorrhages in each quadrant), two venous beading in two or more quadrants and moderate (or “prominent” by the international definition) IRMA in one or more quadrants, known as the 4-2-1 rule.
Very severe NPDR. To diagnose the most severe stage of NPDR, apply the 4-2-1 rule. The “4” in the rule stands for four quadrants of blot/dot hemorrhages, the “2” is two quadrants of venous beading, then finally the “1” is one or more quadrant of IRMA.
Close monitoring is absolutely necessary in these patients because the ETDRS found 52% risk of progression to PDR in one year and a 60% to 75% risk of progression to high-risk PDR in five years.3,12
Proliferative DR. The hallmark of this stage is neovascularization (Figure 3). Neovascularization is defined as either being NVD (on the disc or within one-disc diameter of the optic nerve head) or NVE, elsewhere in the retina. PDR can also include a pre-retinal or vitreous hemorrhage. High-risk PDR is defined as one or more of the following: NVD greater than one-fourth to one-third disc area in size, NVD with a fresh vitreous or pre-retinal hemorrhage or NVE greater than one-half a disc area in size associated with a pre-retinal or vitreous hemorrhage.
 |
| Click chart to enlarge. |
Step 2: Determine if Macular Edema is Present
DME can occur at any stage of retinopathy and can be difficult to observe clinically. In fact, even patients with 20/20 vision can have DME. It is best appreciated with careful slit lamp examination of the macula with a 78 or 90 diopter lens. Historically, clinically significant diabetic macular edema (CSME) was defined by the ETDRS as meeting one of the following criteria:
- Thickening at or within 500µm of the center of the fovea.
- Hard exudates at or within 500µm of the center of the fovea with associated thickening.
- A one-disc diameter (DD) of thickening that is within one DD of the center of the fovea.
Traditional treatment for CSME was grid/focal laser surgery. Previous research showed that using focal laser for patients with CSME is effective at reducing moderate vision loss by 50%.13 Rarely, however, was vision significantly improved by laser therapy. The hope was to stabilize vision and lessen further loss, not necessarily improve it. Over time, the concept of strictly defined CSME has evolved. With the widespread use of OCT, much of the literature now simply subdivides DME into center-involving (CI) when retinal thickening is located within the OCT central subfield zone (1mm in diameter) of the fovea, and non-CI DME when the thickening lays outside the central subfield zone.2,14
So, what central subfield thickness number on an OCT printout should prompt a referral for treatment? The exact number is hard to pinpoint because various studies of patients with diabetes use different center thickness values depending on the type of OCT instrument being used in the study. For example, the VIVID and VISTA studies used a central thickness of 300µm with a Cirrus OCT (Zeiss).15 What happens in reality is that clinicians rely on the OCT’s normative database to determine if the swelling warrants treatment. Clinically, if the center ring of the OCT printout is flagged as abnormally thick, the patient is referred for DME intervention. Some patients with diabetes have relatively thin retinas due to associated neurodegeneration of the retina, so looking for significant change from baseline may be more useful than reliance on ‘color-flagged’ OCT thickness abnormalities.
Routine screening of patients with OCT and FA is not necessary.1,16 However, if you suspect that your patient has macular edema, or their vision is reduced, performing a macular OCT scan would be indicated. FA can be useful in identifying capillary nonperfusion at the macula or enlargement of the foveal avascular zone. It’s important to identify CI-DME because the ETDRS found a 10-fold greater risk of moderate vision loss at one year in these eyes compared to eyes with non-CI DME.3,17
DME has historically been treated with laser. However, multiple clinical trials show that intravitreal anti-VEGF injections work better.18,19 The advantage of injections is being able to improve vision instead of merely stabilizing it. The downside of anti-VEGF treatment is the necessity for repeated injections over a long period of time, which comes with a financial burden and the challenge of attending many office visits. In cases where anti-VEGF injections and additive laser therapy prove to be insufficient, intravitreal steroid injections or sustained-release implants can be considered. While frequently effective, they carry the risk of significant intraocular pressure elevation and unavoidable cataract formation.
What if your patient has non-CI DME? The ETDRS report #19 stated that patients with non-CI DME could be observed closely, which may be preferable to immediate treatment.20 Currently, there is no established evidence for prompt anti-VEGF treatment versus observation versus laser regarding non-CI DME. If the clinician elects to observe, close monitoring of the patient every two to four months is warranted. Also, the discussion of treatment options such as laser or anti-VEGF injections for non-CI DME should be clearly documented in the chart.
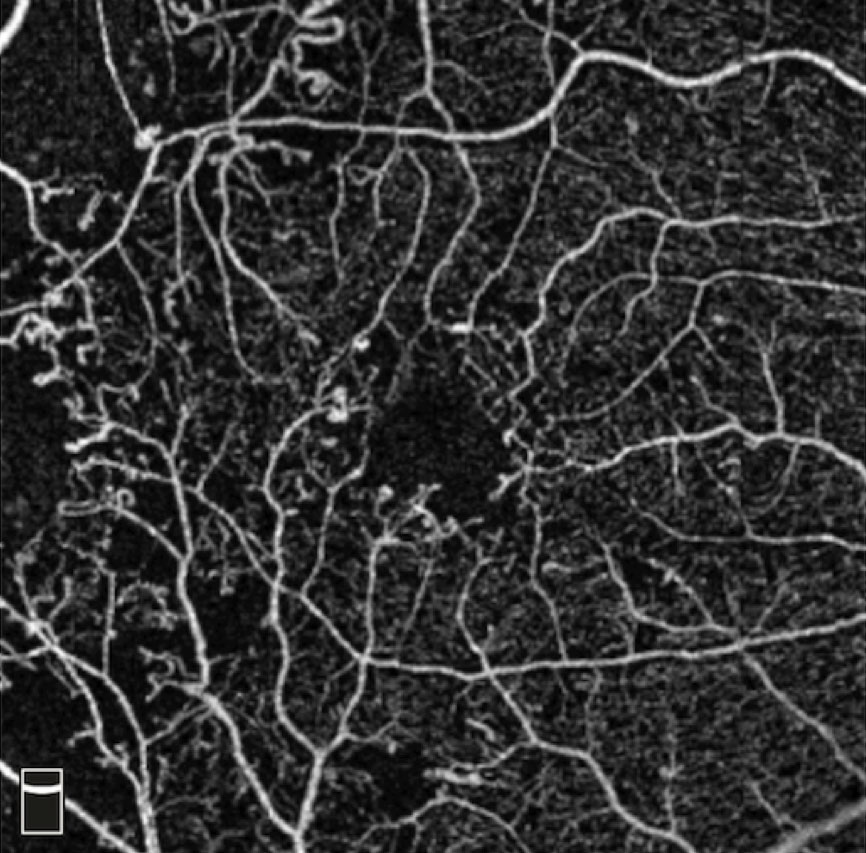
The best approach for subclinical DME and center-involving DME without visual loss is not clearly defined in the literature. Subclinical DME describes a situation where macular thickening is present centrally on the OCT, yet thickening of the macula is not noted on clinical examination (Figure 4). The DR Clinical Research (DRCR) Network’s Protocol G looked specifically at the issue of subclinical DME, and found that up to one-half of eyes in the study with subclinical DME progressed to clinically apparent DME within two years.21
In patients with CI DME and good vision (20/25 or better), DRCR Network’s Protocol V research is still ongoing and is designed to answer the question if observation or prompt treatment is the best course of action.22 Management of patients with these subcategories of macular edema will depend on your comfort level with observation vs. referral. If patients are not immediately referred to a retinal specialist for management, they should be seen every two to four months for close monitoring.
 |
| Fig. 2. This OCT-A shows a patient with microaneurysms and capillary dropout. Click to enlarge image. Photo: Julie Rodman, OD |
Consider Location
Certain retinal locations are more subject to high-risk characteristics of DR than others. Studies have found that the majority of NVE lesions are located inferonasal to the optic disc and along the superior arcades, while NVD has a predilection for the superior temporal rim.23,24 While DR still exists outside these locations, it can be helpful to focus your fundus examination on these areas, especially in patients with small pupils or poor fixation. Remember, fundus photography can also be quite valuable in patients prove difficult to examine, since the photographic images can be magnified and closely scrutinized for subtle signs of retinopathy. This is also another area where OCT-A may prove useful for detecting NVD or NVE at the vitreo-retinal interface.
Ultra-widefield imaging allows for high resolution of the peripheral retina and can be performed using standard color photography, FA, autofluorescence and indocyanine green angiography. Widefield imaging typically allows up to 200 degrees of the retina to be photographed in a single image versus a 75-degree view of the fundus when using the conventional seven standard fields proposed in the ETDRS.25
Careful examination of the peripheral retina is important in diabetic patients because it can detect early signs of non-perfusion that can lead to disease progression. Research has shown a correlation between DME and peripheral retinal ischemia. Many experts feel that the severity of DR may be underestimated when the peripheral fundus is not closely observed and DRCR.net Protocol AA is underway to address the issue more conclusively.25-28
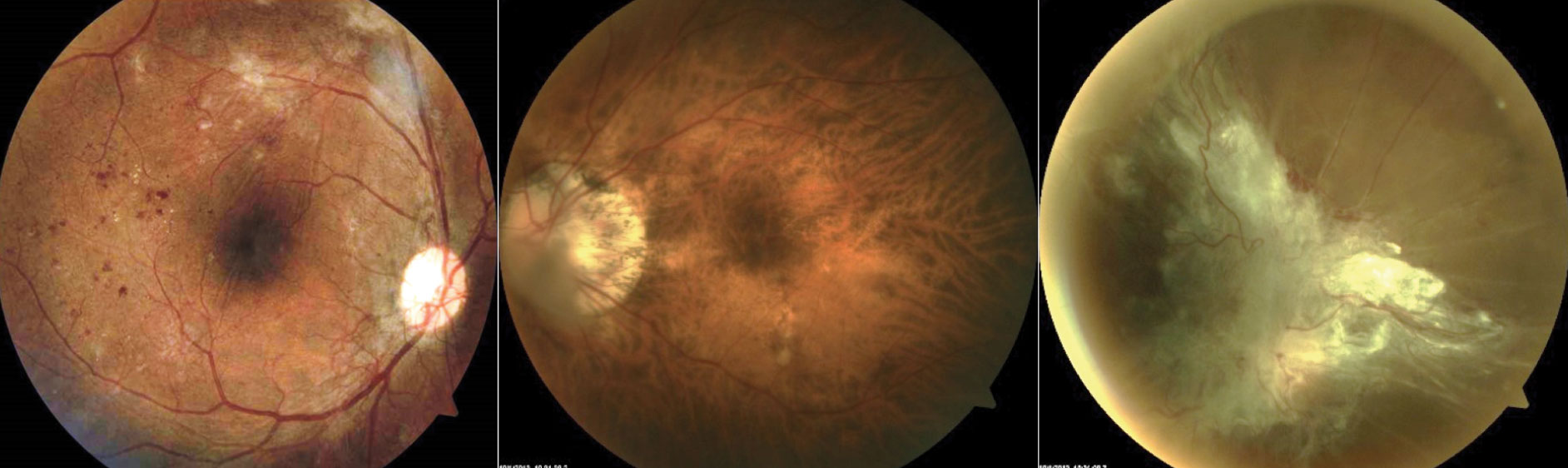 |
| Fig. 3. These fundus images show, from left to right, a patient who has high risk PDR OD: >1/4D-1/3D of NVD inferiorly and NVE superiorly with fibrovascular tissue causing vertical retinal folds through macula; a patient with high myopia (-11.50DS), which can be protective against DR; large posterior vitreous detachment in front of the optic nerve head in the right eye of a patient who failed to seek treatment for PDR. Click to enlarge image. |
Systemic Risk Factors
In addition to the location and current stage of retinopathy, multiple systemic factors influence the follow-up schedule for a diabetic patient. A careful review of systems can reveal important information to better define the patient’s risk of onset and progression of DR.
Non-modifiable risk factors for DR include duration of diabetes, age, ethnicity, genetics and pregnancy.29 Because the duration of diabetes is one of the strongest predictors of the development and progression of DR, it is important to note how long your patient has had diabetes.29-32 American epidemiological studies show African Americans, Latino Americans and Native Americans have the highest rates of visual impairment and blindness from DR.33-35
If your diabetes patient becomes pregnant, she should undergo a complete eye examination soon after conception or early during the pregnancy and every trimester, particularly if DR was more than mild at baseline. However, women who develop gestational diabetes do not require an eye examination during pregnancy.1
 |
| Fig. 4. This fundus image shows clinically significant macular edema with best-corrected visual acuity of 20/20. |
Step 3: Decide to Refer for Treatment or Monitor
Patients with minimal/mild DR can be monitored annually because only 5% to 10% of these patients will progress to more advanced stages of retinopathy over the course of one year.1-3,30,36,37 Patients with moderate NPDR, in the absence of macular edema, should return every six to twelve months for a close examination of the fundus.1-3 Moderately severe NPDR patients should be monitored every two to four months, as up to 27% of patients with moderately severe DR will develop PDR in one year.11,38 If you decide to refer your patient for moderately severe NPDR, consult with your local retina specialist to ascertain their treatment preferences. In regards to DME, clinicians should make a referral to a retinal specialist within two weeks to treat CI macular edema
Management of PDR patients is straightforward: any patient with either PDR or high-risk PDR should be referred promptly to a retinal specialist for treatment, which typically consists of anti-VEGF injections and panretinal laser photocoagulation (PRP), either individually or in combination.
Severe cases involving vitreous hemorrhage and tractional retinal detachment often require surgical intervention. Consult with the specialist within 48 hours for high risk PDR and within one week for PDR. The DR Study found that with prompt treatment of high-risk PDR with PRP, the risk of severe vision loss (<5/200) was reduced by more than 50%.39,40
 |
| Click to download this referral form. |
Step 4: Educate and Communicate
A key component to managing patients with diabetes is appropriate communication to those involved in the patient’s care. This starts with a chairside discussion. Only half of all those with diabetes have a yearly eye exam.41 To improve this statistic, our job as eye care providers is to educate our patients and the community at large. Our patients must understand DR, and its sight threatening complications. Educational resources include handouts, exam room posters, and incorporation of diabetes education within your website or social media accounts. Handouts can be self-designed or taken from pre-existing resources like the National Eye Institute or American Optometric Association.
Regardless of your approach, it is important to emphasize modifiable risk factors:
- Glycemic control. Goal HbA1c in Type II diabetes varies depending on age and comorbidities. The American Diabetes Association recommends below 7.0% for most non-pregnant adults.42
- Smoking status. The exact relationship between smoking and DR progression is controversial. Regardless, from a public health standpoint, patients should be advised to lead a healthier lifestyle by not smoking. Encourage patients to kick the habit by providing them with local and state cessation programs. Ask them if they want to quit and focus efforts on those who answer affirmatively.
- Hypertension. Stress an ideal blood pressure below 130/80mm Hg. Understand that ACE inhibitors or angiotensin receptor blocking agents are standard of care for hypertension in diabetes, and have been shown to have a retino-protective effect in several studies.43
- Obesity. Emphasize weight loss through physical exercise and proper nutrition. Suggest a plant-based diet and elimination of processed foods and soft drinks.
- High cholesterol. Optimize cholesterol levels with a diet low in saturated and hydrogenated fats and relatively high in poly- and monounsaturated fats that can raise high-density lipoproteins (HDL). Current literature shows that statin treatment for dyslipidemia reduces the risk of DR in Type II diabetes, as does the triglyceride lowering agent fenofibrate.44-46
- Poor sleep habits. Getting six to nine hours of uninterrupted sleep each night reduces the risk of diabetes. Remember also that untreated obstructive sleep apnea (OSA) can worsen DR.47 Encourage diabetic patients with OSA symptoms to have a sleep study.
- Vitamin D. Encourage patients to get outside. Vitamin D deficiency has been linked to diabetes and to increased risk of DR.48
- Nutrition. Consider a science-based nutritional supplement for those with DR.
- PCP/Endocrinologist. Communication must also occur with the patient’s primary care doctor, endocrinologist, or both, after each diabetes-related office visit. Our preference is to send a one-page report focusing on important exam details, specifically visual acuity, blood pressure, presence of DR, level of hypertensive retinopathy (if applicable) and treatment plan. Most electronic health record systems can tailor a template for you. If findings are atypical, or if you are suggesting the patient needs additional lab or diagnostic workup, a formal letter is indicated. A letter needs to appropriately address the target audience, include only pertinent information, follow an organized format and have a clear message. Often, letters contain extraneous information that dilutes the salient points. What needs to be included in a formal letter?
- Brief patient history. This includes the reason for communication and pertinent background information about the patient’s complaint, condition(s) and medical history.
- Objective testing. Here is where providers have to focus on what is pertinent information. Best-corrected VA should always be included, as it is understood universally among all provider types. Refraction, though, is almost never necessary to include. Specifically, focus on the dilated fundus examination findings and the results of pertinent ancillary testing such as OCT. Non-ophthalmic providers have stressed that they prefer not to receive communications containing ophthalmic acronyms (e.g., NPDR, OD/OS/OU), so use formal diagnostic terminology.
- Assessment and recommendation. What is your diagnosis and treatment plan? Make it clear whether you are following up with the patient and when, or if you are referring the patient for specific management. Formal letters, though more time-consuming, are an excellent means to provide thorough patient care and build better relationships with our medical colleagues. Whether you choose a short report, formal letter, or phone call will depend upon the clinical scenario and urgency of the message.
 |
| Click to download this guide. |
Retina Referral Tips
Most of us already have established relationships with our “go-to” ophthalmology providers for cataract, retina and glaucoma. Once you decide a patient needs retina specialist evaluation and possible treatment for DR, have one of your staff members call to arrange an appointment and verify that the patient’s insurance is accepted. After the patient is scheduled, it is a matter of faxing a short report or copy of the exam record to the retinal specialist. A formal letter is generally unnecessary if the referral is uncomplicated, as retinal specialists will understand all of the ocular findings included in the exam record or brief report.
When should optometrists hold ’em and monitor DR vs. fold ’em and refer for treatment? The paradigm is shifting as new research findings emerge. In the future, it may become standard of care to administer anti-VEGF treatments to reduce the severity of DR rather than to just treat already existing DME or PDR.
Proper management of DR starts with a good understanding of the different stages of retinopathy and the treatment options that are available. Modifiable risk factors for DR need to be discussed with the patient and effective communication with the patient, PCP, endocrinologist and retinal specialist is key. Optometrists should base the decision to monitor or refer on a combination of their knowledge that treatment would benefit the patient and their comfort level monitoring DR.
Dr. Torbit is an associate clinical professor at Indiana University School of Optometry.
Dr. Bedwell is an assistant clinical professor at Indiana University School of Optometry.
Dr. Bollier is a primary care resident at the Indianapolis Eye Care Center at Indiana University School of Optometry.
Dr. Sutton is a clinical professor at Indiana University School of Optometry and Service Chief of the Indianapolis Eye Care Center.
1. American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Guidelines. DR. San Francisco, CA: American Academy of Ophthalmology; 2017. www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp-updated-2017. December 2017. Accessed Feburary 14, 2019. 2. International Council of Ophthalmology Guidelines for Diabetic Eye Care 2017. www.icoph.org/downloads/ICOGuidelinesforDiabeticEyeCare.pdf. January 2017. Accessed Feburary 14, 2019. 3. American Optometric Association. Eye Care of the Patient with Diabetes Mellitus. Evidence-Based Clinical Practice Guideline 2014. www.aoa.org/Documents/EBO/EyeCareOfThePatientWithDiabetesMellitus%20CPG3.pdf. February 7, 2014. Accessed February 14, 2019. 4. Bhavsar KV, Subramanian ML. Risk factors for progression of subclinical diabetic macular oedema. Br J Ophthalmol. 2011;95(5):671-4. 5. Ip M, Zhang J, Ehrlich J. The clinical importance of changes in DR severity score. American Academy of Ophthalmology. 2017;124(5):596-603. 6. Ip M, Domalpally A, Hopkins J, et al. Long-term effects of ranibizumab on DR severity and progression. Arch Ophthalmol. 2012;130(9):1145–52. 7. Ip M, Domalpally A, Sun JK, Ehrlich JS. Long-term effects of therapy with ranibizumab on DR severity and baseline risk factors for worsening retinopathy. Ophthalmol. 2015;122(2):367-74. 8. Wykoff C, Eichenbaum D, Roth D. Ranibizumab induces regression of DR in most patients at high risk of progression to proliferative DR. Ophthalmology Retina. 2018;2(10):997-1009. 9. Slakter J, Schneebaum J, Shah S. Digital Algorithmic DR Severity Scoring System (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc. 2015;113:T9. 10. Staurenghi G, Feltgen N, Arnold J, et al. Impact of baseline DR Severity Scale scores on visual outcomes in the VIVID-DME and VISTA-DME studies. Br J Ophthalmol. 2017;102(7):954-958. 11. Baresegian A, Kotlyar B, Lee et al. DR: focus on minority populations. Int J Clin Endocrinol Metab. 2017;3(1):34-45. 12. Early Treatment DR Study Research Group. Early photocoagulation for DR. ETDRS report number 9. Ophthalmology. 1991;98(5 Suppl):766-85. 13. Early Treatment DR Study Research Group. Photocoagulation for diabetic macular edema. Early treatment DR study report number 1. Arch Ophthalmol 1985;103:1796-806. 14. Tomic M, Vrabec R, Poljicanin T, et al. Diabetic Macular Edema: Traditional and Novel Treatment. Acta Clin Croat. 2017;56(1):124-32. 15. Pieramici D, Singh R, Gibson A, et al. Outcomes of diabetic macular edema eyes with limited early response in the VISTA and VIVID studies. Ophthalmol. 2018;125(7):952-8. 16. Browning D, Fraser C, Clark S. The relationship of macular thickness to clinically graded DR severity in eyes without clinically detected diabetic macular edema. Ophthalmol. 2008;115(3):533-9. 17. Early Treatment DR Study Research Group. Photocoagulation for diabetic macular edema. ETDRS report No. 4. Int Ophthalmol Clin. 1987;27(4):265-72. 18. Heier J, Korobelnik J, Brown D, et al. Intravitreal aflibercept for diabetic macular edema: 148-Week Results from the VISTA and VIVID Studies. Ophthalmol. 2016;123(11):2376-85. 19. Gross J, Glassman A, Liu D, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative DR: A randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138–48. 20. Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to Fluorescein angiographic and other retinal characteristics at baseline: ETDRS Report No. 19. Arch Ophthalmol. 1995;113(9):1144-55. 21. Bressler N, Miller K, Beck R. Observational study of subclinical diabetic macular edema. DR Clinical Research Network. Eye. 2012;26:833-40. 22. Treatment for CI-DME in eyes with very good VA Study (Protocol V). DR Clinical Research Network. clinicaltrials.gov/ct2/show/NCT01909791. Accessed February 15,2019. 23. Ferman S, Leonard-Martin T, Semchyshyn T. The topographic distribution of the first sites of diabetic retinal neovascularization. Am J Ophthalmol. 1998;125(5):704-706. 24. Jansson RW, Frøystein T, Krohn J. Optic disc neovascularization in early stages of proliferative DR. Inves Ophthalmol Vis Sci. 2012;53(12):8246-52. 25. Bae K, Lee J, Kim T, et al. Anterior DR studied by ultra-widefield angiography. Korean J Ophthalmol. 2016;30(5):344-51. 26. Wessel M, Nair N, Aaker G, et al. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96(5):694-8. 27. Silva P, Dela Cruz A, Ledesma M, et al. DR severity and peripheral lesions are associated with nonperfusion on ultra-wide field angiography. Ophthalmol. 2015;122(12):2465–72. 28. Manjunath V, Papastavrou V, Steel D, et al. Wide-field imaging and OCT vs clinical evaluation of patients referred from DR screening. Eye. 2015;29(3):416-23. 29. Scanlon P, Aldington S, Stratton I. Epidemiological issues in DR. Middle East Afr J Ophthalmol. 2013;20(4):293-300. 30. Klein R, Klein B, Moss S, et al. The Wisconsin epidemiologic study of DR X. Four-year incidence and progression of DR when age at diagnosis is 30 years or more. Arch Ophthalmol. 1989;107(11):244-9. 31. Yau J, Rogers S, Kawasaki R, et al. Global prevalence and major risk factors of DR. Diabetes Care. 2012;35(3):556-64. 32. Ting D, Cheung G, Wong T. DR: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44(4):260-77. 33. Barsegian A, Kotlyar B, Lee J, et al. DR: Focus on minority populations. Int J Clin Endocrinol Metab. 2017;3(1):34-45. 34. Varma R, Torres M, Pena F, et al. Prevalence of DR in adult Latinos: the Los Angeles Latino eye study. Ophthalmol. 2004;111(7):1298–306. 35. Munoz B, O’Leary M, Fonseca-Becker F, et al. Knowledge of diabetic eye disease and vision care guidelines among Hispanic individuals in Baltimore with and without diabetes. Arch Ophthalmol. 2008;126(7):968–74. 36. Modjtahedi BS, Theophanous C, Chiu S, et al. Two-year incidence of retinal intervention in patients with minimal or no DR on telemedicine screening. JAMA Ophthalmol. www.researchgate.net/publication/330943022_Two-Year_Incidence_of_Retinal_Intervention_in_Patients_With_Minimal_or_No_Diabetic_Retinopathy_on_Telemedicine_Screening. February 2019. Accessed April 22, 2019. 37. Vitale S, Maguire MG, Murphy RP, et al. Interval between onset of mild non-proliferative and proliferative retinopathy in type 1 diabetes arch Ophthalmol. 1997;115(2):194-198. 38. International Clinical DR Disease Severity Scale, Detailed Table. Authored by American Academy of Ophthalmology. The Eye MD Association. www.icoph.org/resources/45/International-Clinical-Diabetic-Retinopathy-Disease-Severity-Scale-Detailed-Table-.html. October 2002. Accessed February 15, 2019. 39. Photocoagulation treatment of proliferative DR clinical application of DR study (DRS) findings, DRS report number 8. The DR study research group. Ophthalmology. 1981;88(7):583-600. 40. Alvi R, Memon MS, Shera S, et al. Visual outcome of laser treatment in diabetic macular edema: Study from an urban diabetes care center. Pak J Med Sci. 2016;32(5):1229-33. 41. National Eye Institute. Diabetic Eye Disease. nei.nih.gov/nehep/programs/ diabeticeyedisease. Accessed February 12, 2019. 42. American Diabetes Association. 6. Glycemic targets: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S61–S70 43. Wang B, Wang F, Zhang Y, et al. Effects of RAS inhibitors on DR: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015 Apr;3(4):263-74. 44. Kang EY, Chen TH, Garg SJ, et al. Association of Statin Therapy With Prevention of Vision-Threatening DR. JAMA Ophthalmol. 2019 Jan 10. 45. Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–97. 46. Accord Eye Study Group, Chew EY, Ambrosius WT, Davis MD, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233-44. 47. Atlaf Q. Obstructive sleep apnea and retinopathy in patients with Type 2 diabetes. A longitudinal study. Am J Respir Crit Care Med. 2017;196(7):892–900. 48. Luo B, Gao F, Qin L. The Association between vitamin D deficiency and DR in type 2 diabetes: A meta-analysis of observational studies. Nutrients. 2017;9(3):307. |

