It is estimated that glaucoma affects over three million Americans and over 76 million adults over the age of 40 worldwide.1,2 By 2050, glaucoma is expected to impact nearly eight million adults in the United States and over 110 million around the globe.3 Glaucoma is a leading cause of blindness, and the only proven therapy that has been shown to decrease the risk of progression is reducing intraocular pressure (IOP).4,5
Historically, topical medications were the mainstay therapy; now the most effective management involves a continuum of both medical and surgical treatments.6,7 Multiple factors determine which treatment paradigm bests suits each patient. Details such as the disease severity at the time of diagnosis, rate of disease progression, age of the patient, ocular and systemic comorbidities, allergies, previous therapy, cost of treatment, target IOP and other patient-specific issues and preferences must be considered.8
Helping patients understand the disease, set long-term expectations, embrace various therapeutic options and anticipate potential hurdles plays an essential role in successful outcomes; namely, slowing the progression of the disease and reducing vision loss. This article focuses on the specific factors optometrists should address with their patients for the best results with topical therapy.
Price and Acquisition
Cost is one of the most important things to keep in mind to encourage adherence to therapy and patient compliance.9,10 Financial constraints are a real issue many patients face in today’s healthcare system. Data from the National Center for Health Statistics indicates roughly 10% of the US population was uninsured in the first quarter of 2020.11 With the COVID-19 pandemic, these statistics are expected to worsen as loss of jobs means loss of employer-provided insurance.12 Even having insurance or a Medicare plan does not necessarily guarantee an associated drug benefit. Sampling a new drop is only effective if the patient can afford it, let alone locate it at a nearby pharmacy and tolerate its side effects.
 |
|
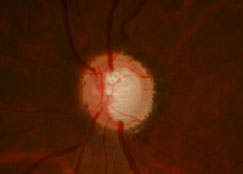
Glaucomatous optic nerve. Photo: The Eye Center at SCO. Click image to enlarge. |
Lifetime medication use is expensive, especially if the disease progresses and the patient requires additional therapy. This factor must be weighed against surgical intervention and the associated set of fees and adverse effects. After taking all costs into consideration, one study found that selective laser trabeculoplasty (SLT) is typically less expensive than most long-term brand name medications.13 Another study reported that SLT effectively maintained IOP in newly diagnosed glaucoma and ocular hypertensive patients over a 36-month period.14 Patients who had SLT were at or below target IOP readings for more follow-ups than their counterparts on topical therapy.14
Prescribing generic medications when possible can also be a less costly alternative. However, the cost of generic medications is set by each individual pharmacy, so price may vary widely, even within the same community. Two optometrists started werx.org to help patients determine the cost analysis of medications across local pharmacies for both name brand and generic medications. A similar feature is offered on www.goodrx.com. Many pharmacies and other organizations (including ScriptSave WellRx) have membership plans that offer opportunities for reduced medication cost. It would be beneficial for prescribers to review local providers to evaluate what ophthalmic drugs may be available and at what cost.
Table 1. Combination Medications Commercially Available in the United States | |
Name (Manufacturer) | Ingredients |
CoSopt/CoSopt PF (Akorn) | timolol maleate 0.5% + dorzolamide hydrochloride 2% |
Combigan (Allergan) | timolol maleate 0.5% + brimonidine tartate 0.2% |
| Simbrinza (Novartis) | brinzolamide 1% + brimonidine tartate 0.2% |
| Rocklatan (Aerie) | netarsudil 0.02% + latanoprost 0.005% |
Additional web-based resources such as www.needymeds.org and www.blinkhealth.com offer ways to assist patients in navigating the cost of their medications, including one-stop access to manufacturer-sponsored patient assistance programs, coupons, rebate programs and even drug delivery. Discussing all of these options with patients can help them understand their choices and how to best afford their care.15
To that end, online formulary search tools can ensure the recommended therapy is cost effective for each patient. Many insurance providers post their medication formularies online through easily accessible platforms. There are also several search engines and associated cell phone apps that offer information on medication coverage for a multitude of insurance providers in a region. Such resources include www.formularylookup.com, lookup.decisionresourcesgroup.com, www.epocrates.com and www.healthcare.gov.16
Table 2. Compounded Combination Medications Available Through ImprimisRx |
brimonidine (0.15%) + dorzolamide (2%) |
timolol (0.5%) + brimonidine (0.15%) + dorzolamide (2%) + latanoprost (0.005%) |
timolol (0.5%) + brimonidine (0.15%) + dorzolamide (2%) |
timolol (0.5%) + dorzolamide (2%) + latanoprost (0.005%) |
timolol (0.5%) + latanoprost (0.005%) |
Insurance considerations and financial concerns can be a limiting factor to medication compliance, yet optometrists spend little time discussing the cost of therapy with their patients—a barrier that we can easily overcome.17,18 Even if the patient is able to afford and obtain the prescribed medication, non-adherence is one of the most important and costly worldwide healthcare problems.19 A recent study demonstrated that roughly half of patients who start topical glaucoma therapy are noncomplaint or have completely discontinued their prescribed medications by six months.20 An additional study analyzed dispensing records to determine the degree of adherence for glaucoma medication refills at community pharmacies and found that over a 12-month period, only about 57% of patients had the proper amount of medication to last the year.19 In addition to cost and accessibility, several other factors can help us avoid these outcomes.
Routine and Regimen
 |
|
The Autodrop Eye Drop Guide (Maddak) can help patients with drop instillation. Click image to enlarge. |
Patients often have difficulty fitting glaucoma drops into their established routine due to forgetfulness.21-23 Asking patients about the details of their daily routine, such as when they wake up, work, eat and sleep, can assist in drop integration. When a patient’s lifestyle needs dictate an atypical dosing schedule, optometrists must understand how to best accommodate the patient. For example, patients who have evening or overnight work schedules may benefit from altering the typical nightly dosing regimen and instead take prostaglandin analogs in the morning in conjunction with their sleep schedule to maintain IOP. One study showed that mean 24-hour IOP control was equivalent when comparing travoprost dosed in the morning vs. in the evening.24
If a patient is already compliant with other regimens such as oral medications and self-care routines such as brushing teeth, placing the eye drops near their medication or toothbrush may improve compliance with therapy. To optimize the 12- or 24-hour effect of the bulk of glaucoma drops, setting reminders can be beneficial. Cell phone alarms, calendar alerts and app reminders are all options. A study showed that 72.9% of glaucoma patients expressed interest in using an app, one of the more popular routes that we can take advantage of.25 Along with these recommendations, pharmacy auto-refills or mail orders are also advised so that patients do not run out of their medications.
The complexity of the dosing regimen can also affect adherence. Simply adding a second eye drop to the regimen can increase dosing errors by nearly 400%.15,26 There are a multitude of combination agents available commercially and through compounding pharmacies that can make treatment coordination and adherence easier for patients (Tables 1 and 2).27
Adherence to follow-up appointments is still necessary to complete diagnostic testing to ensure the patient’s daily routine and glaucoma medication are sufficient in disease control, and pre-appointing follow-up exams helps ensure patients return for their on-going assessments.
Instillation
Administering the drop itself can be exceedingly difficult for some patients with limited mobility or arthritis. Poor aim, inability to extend their neck and dexterity issues can all add up to cause an undesirable result. In fact, one study found that nearly nine out of every 10 glaucoma patients were unable to instill eye drops correctly.28 Another found that patients administer an average of seven drops before they feel confident in the instillation process.29 The author of the latter study published a video titled “Glaucoma Drop Operator Error” that can be found at eyetube.net/videos/glaucoma-drop-operator-error.
Finally, other researchers concluded that despite excellent confidence in their ability, patients rarely administer topical ophthalmic drops without touching the bottle to their eye, which increases risk of infection.30 Punctal occlusion or eyelid closure for three to five minutes post-instillation is an important final step of the process that many patients miss, as it can have the intended effect of increasing ocular penetrance and decreasing systemic absorption.31
 |
|
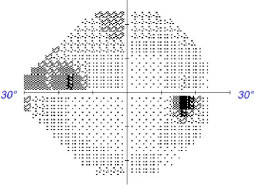
Glaucomatous visual defects tend to appear as negative scotomas, but rather than presenting as dark or blurred, the brain creates an inaccurate but believable image in the defective visual field using surrounding retinal images. By late stages of the disease, the brain no longer receives sufficient visual input to compose an image. Click image to enlarge. |
In-office training, including watching the patient administer a drop to their eye, can help mitigate and correct many instillation mistakes. To aid in the instillation process, there are several different types of auto drop mechanisms and guides on the market.32 Some are pharmaceutical company-specific, and others can be obtained online for use with a variety of different-sized bottles. One study proposed using an alternate instillation technique into the medial canthal area over a closed eyelid.33 The investigators observed similar reductions in IOP with this method compared with direct instillation onto the cornea.33 Directing your patient to resources on drop administration such as www.glaucoma.org and www.aoa.org can be beneficial in giving them a reference point and tips on how to apply the medication to the eye.34,35
Complications
Side effects of medications are another common barrier to drug adherence encountered by patients with glaucoma. These patients overcome the obstacles of obtaining and instilling the drop only to be thwarted by intolerable adverse events, which can be temporary or persistent. Stinging, redness and burning are common sequelae of drop instillation. Storing the drop in the refrigerator can help alleviate these complaints and helps the patient with proper administration onto the ocular surface as the drop is easier to detect.34
Another consideration is the effect these medications have on pre-existing ocular conditions or diseases, especially when it comes to dry eye.36 Controlling and treating the underlying disease concurrently with glaucoma treatment can help prevent ocular discomfort. Preservatives that are inherent in topical therapy can exacerbate discomfort, specifically benzalkonium chloride (BAK).37 To alleviate this dilemma, use of preservative-free medication—Zioptan (tafluprost, Akorn), CoSopt PF (dorzolamide hydrochloride-timolol maleate, Akorn), Timoptic in Ocudose (timolol maleate, Bausch + Lomb)—or alternatives to BAK-preserved medication—Travatan Z (travoprost, Novartis), Alphagan P (brimonidine, Allergan), Xelpros (latanoprost, Sun Pharmaceutical)—are recommended, especially for those who require additional therapy.38
Requesting the patient bring their medication bottle with them to subsequent appointments may be necessary when attempting to decipher patient complaints and confirm they are taking the correct drug in the first place.
Patient Education
Upon prescribing a new medication, effective education can help guide patient expectations moving forward.39 Patients may notice mild momentary blurring or clearing of their vision from the effect of the drop on the ocular surface and assume it is a problem with the efficacy of the medication rather than a temporary adverse effect.21 We can resolve this popular misconception with clear communication prior to the patient beginning their regimen. If side effects persist and are intolerable, however, patients must understand the importance of calling the office, as the medication may be substituted.
 |
|
|
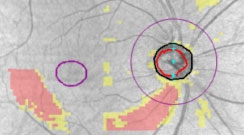
| The PanoMap feature on the Zeiss Cirrus OCT (top) exhibits thinning of both the retinal nerve fiber layer and ganglion cell layer-inner plexiform layer complex with a corresponding visual field result (bottom) showing a superior nasal step defect. Click image to enlarge. |
Glaucoma management can be fraught with miscommunication, and health literacy plays an important role in patient compliance and disease treatment.22 A study found that 44% of patients in an urban American setting did not have an acceptable idea of what the term “glaucoma” means and that 30% were unsure as to why they were taking medications in the first place.40 Without appropriate understanding of their disease or its management, patients lose motivation and can progress to advanced stages of glaucoma due to poor adherence to treatment and follow-up.41
Many individuals may not even want to pursue treatment until symptoms present; hence, doctors must advise that the associated vision loss is irreversible. Patients should be reminded that treatment is not geared toward prevention of glaucoma, but rather prevention of vision loss. Along these lines, patients should not get into the habit of assuming a lower IOP reading equates to adequate control and stop using their drops or only use them prior to an appointment.
Patients should also be informed of their test findings and rate of progression, when applicable. Perceptual visual field loss can be vastly different from what doctors observe on automated visual field testing. One study found 45% of patients were completely unaware of the true nature of their vision loss.42 The survey showed that patients do not have tunnel vision or perceive black spots in their vision; rather, they see blurred patches. Patients should be informed that glaucoma is slowly progressive and they will likely be asymptomatic until advanced visual field loss occurs. Hence the importance of treatment continuation despite perceived subjective improvements.
Some patients rarely participate in preventive care or routine screenings or physicals to begin with. These patients may only seek medical care if any problems or symptoms that do arise fail to improve. On the other hand, some patients are too discouraged to seek out medical care, whether due to trust issues with the physician or unfavorable practice conditions.22,43 If one optometrist discovers glaucomatous risks or signs when a previous provider did not, the patient may question their judgment. Patients may also assume their glaucoma is similar to an acquaintance’s, but glaucoma status varies based on type of glaucoma, rate of progression and treatment history.
Written instructions as a follow-up to verbal education can help alleviate misunderstandings the patient may encounter. A study showed that even after a patient has been successful with their drug regimen for over a year, continued education and positive reinforcement have ongoing benefits.44 Another confirmed positive reinforcement increases short-term improvements in measures regarding adherence.15 As optometrists, it is our job to continuously educate our glaucoma patients on the purpose of their therapy and follow-ups, and the risks of abstinence and progressive visual loss.23,45
Takeaways
Glaucoma patients are in it for the long haul. Place an emphasis on clear, direct instructions to aid in manageable, patient-friendly care. Be supportive and empathetic while actively listening and providing solutions to patient concerns regarding possible barriers to effective treatment. Advocating for your patient and helping them overcome the challenges they may face will assist in ensuring a successful partnership in their long-term care.
Dr. Dorkowski is an associate professor at the Southern College of Optometry (SCO). Clinically, he works in the advanced care ocular disease and the adult primary care clinics and is coordinator of the nursing home and assisted living programs at the Eye Center at SCO. Dr. Ragha is a clinical instructor at SCO. She sees patients and supervises students in a clinical setting at the Eye Center, in the primary care, ocular disease and fluorescein angiography departments, and at the Focal Point at Crosstown Concourse in Memphis. Dr. Sanderson is an associate professor and the assistant director of residency programs at SCO. The authors have no financial interests to disclose.
1. Vajaranant TS, Wu S, Torres M, et al. The changing face of primary open-angle glaucoma in the United States: demographic and geographic changes from 2011 to 2050. Am J Ophthalmol. 2012;154(2):303-14. 2. Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-90. 3. Vajaranant TS, Wu S, Torres M, et al. A 40-year forecast of the demographic shift in primary open-angle glaucoma in the united states. Invest Ophthalmol Vis Sci. 2012;53(5):2464-6. 4. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901-11. 5. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221-34. 6. European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition - Part 1. Br J Ophthalmol. 2017;101(4):1-72. 7. Preferred practice patterns committee GP. Chicago, IL: American Academy of Ophthalmology; 2010. 8. Fingeret M. Optometric clinical practice guideline, care of the patient with open angle glaucoma. St. Louis, MO: American Optometric Association; 2010. 9. Tsai JC, McClure CA, Ramos SE, et al. Compliance barriers in glaucoma: a systematic classification. J Glaucoma. 2003;12(5):393-8. 10. Feldman RM, Cioffi GA, Liebmann JM, et al. Current knowledge and attitudes concerning cost-effectiveness in glaucoma pharmacotherapy: a glaucoma specialists focus group study. Clin Ophthalmol. 2020;14:729-39. 11. Cohen RA, Terlizzi EP, Cha AE, et al. Health insurance coverage: early release of estimates from the National Health Interview Survey, January-June 2020. National Center for Health Statistics. February 2021. www.cdc.gov/nchs/data/nhis/earlyrelease/insur202102-508.pdf. Accessed March 11, 2021. 12. McDermott D, Cox C, Rudowitz R, et al. How has the pandemic affected health coverage in the U.S.? KFF. December 9, 2020. www.kff.org/policy-watch/how-has-the-pandemic-affected-health-coverage-in-the-u-s/. Accessed March 11, 2021. 13. Seider MI, Keenan JD, Han Y. Cost of selective laser trabeculoplasty vs topical medications for glaucoma. Arch Ophthalmol. 2012;130(4):529-30. 14. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393(10180):1505-16. 15. Robin AL, Muir KW. Medication adherence in patients with ocular hypertension or glaucoma. Expert Rev Ophthalmol. 2019;14(4-5):199-210. 16. Davison R. Making drug formulary search tools better for patients. The Catalyst. September 7, 2016. catalyst.phrma.org/making-drug-formulary-search-tools-better-for-patients. Accessed March 11, 2021. 17. Slota C, Davis SA, Blalock SJ, et al. Patient-physician communication on medication cost during glaucoma visits. Optom Vis Sci. 2018;95(6):554. 18. Rylander NR, Vold SD. Cost analysis of glaucoma medications. Am J Ophthalmol. 2008;145(1):106-13. 19. Feehan M, Munger MA, Durante R, et al. Adherence to glaucoma medications over 12 months in two US community pharmacy chains. J Clin Med. 2016;5(9):79. 20. Nordstrom BL, Friedman DS, Mozaffari E, et al. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598-606. 21. Taylor SA, Galbraith SM, Mills RP. Causes of non-compliance with drug regimens in glaucoma patients: a qualitative study. J Ocul Pharmacol Ther. 2002;18(5):401-9. 22. Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122(7):1308-16. 23. MacKean JM, Elkington AR. Compliance with treatment of patients with chronic open-angle glaucoma. Br J Ophthalmol. 1983;67(1):46-9. 24. Konstas AGP, Mikropoulos D, Kaltsos K, et al. 24-Hour intraocular pressure control obtained with evening- versus morning-dosed travoprost in primary open-angle glaucoma. Ophthalmology. 2006;113(3):446-50. 25. Waisbourd M, Dhami H, Zhou C, et al. The Wills Eye glaucoma app: interest of patients and their caregivers in a smartphone-based and tablet-based glaucoma application. J Glaucoma. 2016;25(9):e787-91. 26. Robin AL, Novack GD, Covert DW, et al. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;144(4):533-40. 27. ImprimisRx. www.imprimisrx.com/search/ophthalmology. Accessed March 11, 2021. 28. Gupta R, Patil B, Shah BM, et al. Evaluating eye drop instillation technique in glaucoma patients. J Glaucoma. 2012;21(3):189-92. 29. Robin AL. Instilling drops. Glaucoma Today. September 2010. [Epub ahead of print]. 30. Stone JL, Robin AL, Novack GD, et al. An objective evaluation of eyedrop instillation in patients with glaucoma. Arch Ophthalmol. 2009;127(6):732-6. 31. Flach AJ. The importance of eyelid closure and nasolacrimal occlusion following the ocular instillation of topical glaucoma medications, and the need for the universal inclusion of one of these techniques in all patient treatments and clinical studies. Trans Am Ophthalmol Soc. 2008;106:138-45. 32. Davies I, Williams AM, Muir KW. Aids for eye drop administration. Surv Ophthalmol. 2017;62(3):332-45. 33. Freddo TF, Ho DY, Steenbakkers M, et al. Validation of a more reliable method of eye drop self-administration. Optom Vis Sci. 2020;97(7):496-502. 34. Gudgel DT. How to put in eye drops. American Academy of Ophthalmology. March 10, 2021. www.aao.org/eye-health/treatments/how-to-put-in-eye-drops. Accessed March 11, 2021. 35. Robin AL. Glaucoma eye drops: suggestions on use. Glaucoma Research Foundation. October 29, 2017. www.glaucoma.org/treatment/glaucoma-eye-drops-suggestions-on-use.php. Accessed March 11, 2021. 36. Zhang X, Vadoothker S, Munir WM, et al. Ocular surface disease and glaucoma medications: a clinical approach. Eye Contact Lens. 2019;45(1):11-8. 37. Rosin LM, Bell NP. Preservative toxicity in glaucoma medication: clinical evaluation of benzalkonium chloride-free 0.5% timolol eye drops. Clin Ophthalmol. 2013;7:2131-5. 38. Yadgarov A, Garg RA. Preservative-free alternatives. Glaucoma Today. November/December 2016. [Epub ahead of print]. 39. Budenz DL. A clinician’s guide to the assessment and management of nonadherence in glaucoma. Ophthalmology. 2009;116(11):S43-7. 40. Costa VP, Spaeth GL, Smith M, et al. Patient education in glaucoma: what do patients know about glaucoma? Arq Bras Oftalmol. 2006;69(6):923-7. 41. Miller NH. Compliance with treatment regimens in chronic asymptomatic diseases. Am J Med. 1997;102(2A):43-9. 42. Crabb DP, Smith ND, Glen FC, et al. How does glaucoma look?: patient perception of visual field loss. Ophthalmology. 2013;120(6):1120-6. 43. Taber JM, Leyva B, Persoskie A. Why do people avoid medical care? A qualitative study using national data. J Gen Intern Med. 2015;30(3):290-7. 44. Curtis C, Lo E, Ooi L, et al. Factors affecting compliance with eye drop therapy for glaucoma in a multicultural outpatient setting. Contemp Nurse. 2009;31(2):121-8. 45. McDonald JE, Dickinson JK. A novel approach to helping people with glaucoma use their drops routinely. Optom Vis Sci. 2019;96(5):331-4. 1. |