Annual Cornea ReportCheck out these articles: Corneoscleral Concerns: Trouble at the Border Skip the Scalpel? A Medical Approach to Endo Recovery |
Microbial keratitis is a generally painful and sight-threatening condition often associated with ocular trauma, ocular surface disease and contact lens wear.1,2 While the spectrum of pathogens and predisposing factors varies with geography and climate, the condition remains a prominent cause of ocular morbidity.1,3,4 Approximately 30,000 cases occur annually in the US, the preponderance of which are of bacterial origin.5 Fungal and Acanthamoeba-based infections are more rare but can be even more debilitating. Viral corneal infections are predominantly herpetic in origin and more easily recognized clinically.
Traditional first-line therapy of infectious ulcers involves the use of empiric treatment with topical antibiotics.4,6-8 The role of microbial culture remains perplexing for many practitioners who wonder if they should withhold therapy until results are in, and the use of certain adjunctive therapies, such as topical corticosteroids, is controversial.4,8
Let’s review the detailed management of microbial keratitis—emphasizing bacterial patterns of infection—including differential diagnosis, culture, treatment strategy and the role of adjunctive therapies.
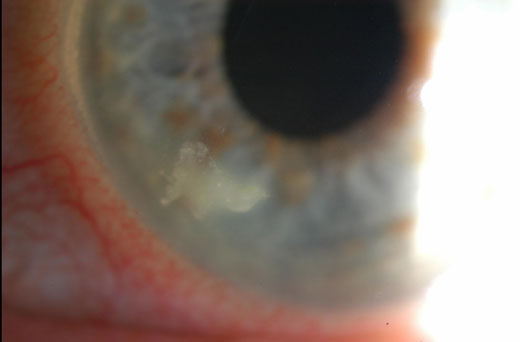 |
This patient developed bacterial keratitis from sleeping in their contact lenses. Click image to enlarge. |
Diagnosing Infectious Keratitis
When a patient presents with a corneal ulcer, judicious consideration of clinical signs and symptoms is paramount in order to discern the etiology and begin appropriate therapy. Clinical signs associated with bacterial origin may include epithelial defects overlying a single stromal infiltrate, indistinct infiltrate edges, corneal edema with white cell infiltration of nearby stroma, anterior chamber reaction, hypopyon, inferior corneal location, older patient age and—though rarely seen—wreathe infiltrates and epithelial plaques.1,6,9-11
According to a review of 300 bacterial keratitis cases, risk factors for bacterial corneal ulcers include, in order of descending importance: contact lens wear, ocular surface disease, acute corneal trauma and corneal surgery.1 Additional risk factors include systemic diseases, immunosuppression and lack of prior topical antibiotic use.1,9,11 Gram-positive microbes are predominantly cultured from bacterial ulcers, particularly in instances of ocular surface disease and corneal trauma.1, 12 Eye care practitioners, however, identify gram-negative Pseudomonas keratitis more readily, which is often associated with less defined, suppurative infiltrates.1,12 Further defining characteristics of gram-negative keratitis include severe anterior chamber inflammation, greater size of infiltrates and more rapid progression.1
While proper classification of other bacterial keratitis strains has been modest, practitioners accurately discriminate bacterial ulcers from their fungal counterparts 66% of the time.6,12 Clinical features associated with fungal keratitis include the following:6,9,10
• satellite lesions
• infiltrates with feathery, fluffy, irregular or serrated margins
• dry, raised or necrotic infiltrates
• infiltrates pigmented with a color other than yellow
• endothelial rings
• longer history of symptoms
Compared with bacterial etiology, fungal ulcers show no significant difference in occurrence of keratic precipitates, immune rings, radial keratoneuritis, endothelial plaques or anterior chamber reactions.10
Although corneal scrapings rarely yield parasitic organisms such as Acanthamoeba, a greater number of clinically differentiating features exist for Acanthamoeba keratitis (AK) relative to bacterial and fungal etiologies.9 Clinical signs characteristic of AK include pseudodendrites, perineural infiltrates, ring-shaped infiltrates, a predilection for the corneal epithelium early in the disease course, younger age, longer duration of symptoms prior to initiation of treatment and history of prior topical antibiotic use.9 Of note, although ring infiltrates have been documented for fungal and bacterial ulcers (as well as herpetic disease), their presence is significantly more indicative of late-presenting Acanthamoeba etiology.9,12 Conversely, satellite lesions—a feature often noted for fungal keratitis—occur at a similar frequency in both Acanthamoeba and fungal infections.9
When to Culture
Once an infectious corneal ulcer is identified, it is imperative to promptly select an effective treatment in order to relieve symptoms and ensure best visual outcomes—and therein lies the problem. Which one? Matching drug efficacy to the infectious organism can yield better outcomes than just choosing a broad-spectrum antibiotic and hoping for the best.
Studies have shown that clinicians aren’t as accurate in identifying uncommon organisms or infectious ulcers that present without the classic characteristics, with many practitioners demonstrating difficulty in discriminating fungal from bacterial ulcers solely based on clinical appearance.10,12 Corneal specimen culture is the gold standard for identifying etiological origins of ulcers.6-8,12,13 Culturing yields information that can help modify treatment of ulcers refractory to empiric therapy, prevent progression of corneal ulcers, reduce antibiotic resistance and minimize medication toxicity from superfluous medications.7,11 For example, in cases of AK, proper treatment is often delayed due to misdiagnosis, and the use of specimen culture may help avoid a long and toxic treatment course.9
Although some sources have advocated for culture of all infectious corneal ulcers, current staining and culturing methodologies have several limitations, including low sensitivity in bacterial, fungal and viral keratitis cases; protracted turnaround time; low bacterial yield during culture; and time, cost and availability limitations.7,12,14,15 Furthermore, in many instances, culture results do not change clinical management; most cases of bacterial keratitis resolve with empiric broad-spectrum fluoroquinolone therapy initiated either in the absence of microbial culture or prior to receipt of culture results.6,7,11,12,16 Given these limiting factors, a selective approach for culturing has been suggested.7,11,14
According to the current bacterial keratitis Preferred Practice Pattern published by the American Academy of Ophthalmology, smears and cultures of infectious corneal ulcers are recommended when any of the following circumstances are present:11
• size >2mm and centrally located
• accompanied by significant stromal melting
• unresponsive to empiric antibiotic therapy
• chronic, multiple or diffuse in number of infiltrates
• accompanied by characteristics suggestive of amoebic, mycobacterial or fungal infection
• found in eyes with a history of corneal surgery
It is important to note that, although most cases of infectious keratitis are treated empirically, the recovery of organisms via culture may be more difficult to perform if treatment has already been initiated.14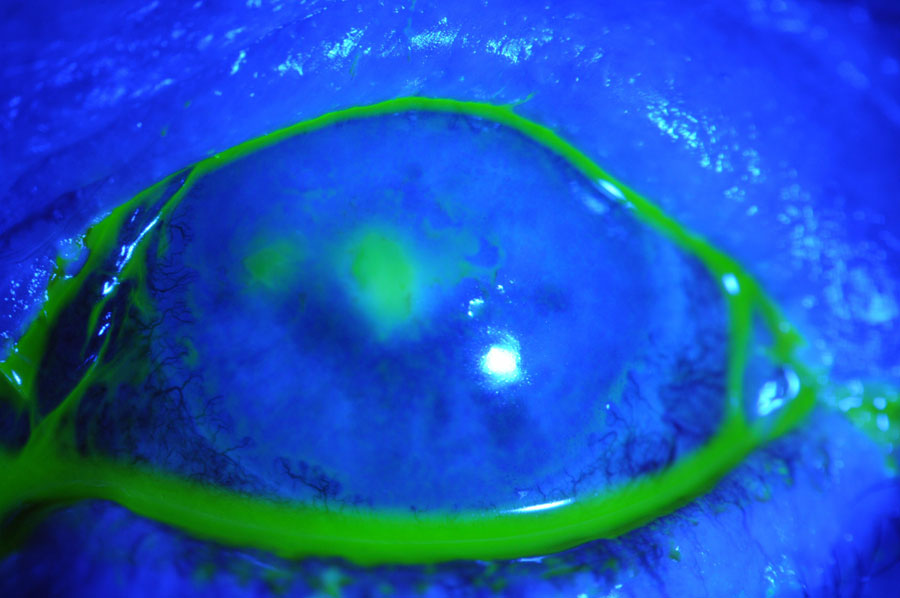
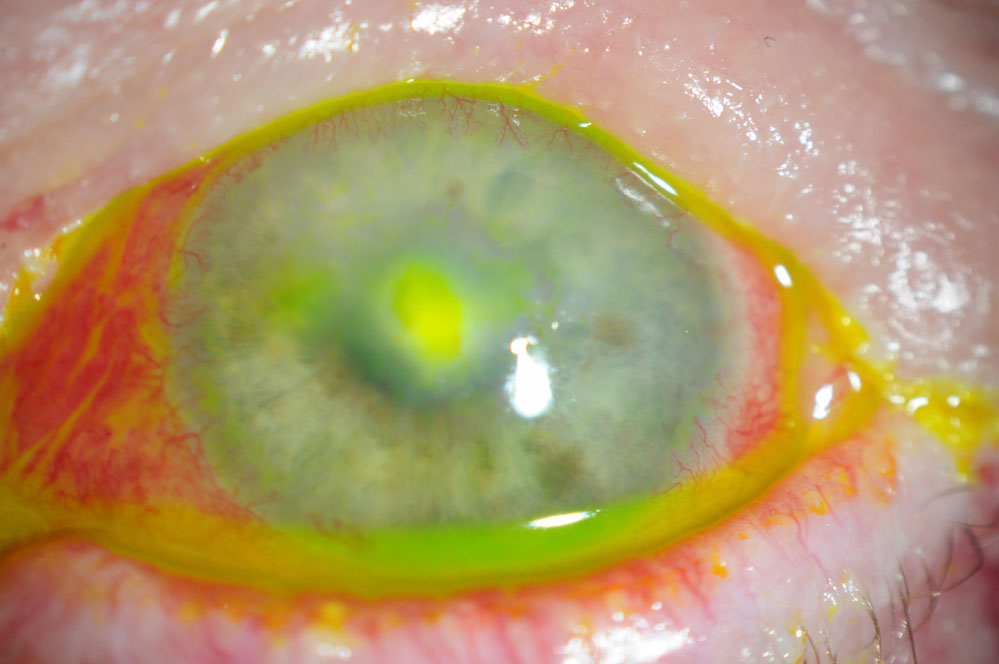 |
This 56-year-old female had a persistent epithelial defect secondary to trichiasis (caused by squamous cell carcinoma lid surgery). She wore a bandage contact lens for pain and developed an ulcer. Click images to enlarge. |
How to Culture
Appropriate culturing technique is critical to maximize yield of organisms (the cornea has a relatively low pathogenic load) and a positive culture. Growth and identification of microorganisms from corneal ulcers may occur in as few as 40% to 60% of cultured cases.6 To increase probability of recovering sufficient microorganisms for a positive culture, multiple samples should be collected and inoculated on various growth media.15
During the procedure, a topical anesthetic is instilled, preferably proparacaine due to its lower bactericidal properties relative to other anesthetics, such as tetracaine.11,14,17 If available, non-preserved topical anesthetic may be used to improve culture yield, and sterile gloves are recommended.11,14,17 The patient should be situated within the slit lamp, as culture is best performed under magnification.11,14,17
A scrape of the corneal ulcer is obtained at the base and advancing edge of the infiltrate from the periphery into the center.11,14,17 Avoid purulent material, as it is less likely to yield sufficient microbial load. However, cultures of contact lenses—cases and solutions— may provide useful information.11,17 Recommended tools for scraping include a 21-gauge needle, a sterilized spud or blade, jeweler’s forceps, a calcium alginate swab or a heat-sterilized Kimura platinum spatula.11,14,17
The material is first smeared onto glass slides if performing gram stain or potassium hydroxide mount, and then inoculated directly onto appropriate agar plates and liquid media (transport media may be used if direct inoculation is not possible).11,14,17 Scrapings should be repeated several times with fresh blades or flame sterilization of the same blade.14,17 Keep cultures at room temperature or incubated if possible, and promptly transport to a laboratory with appropriate labels.11,14,17
Lab reports may come back in as little time as several hours for gram stains, one to two days for bacterial growth or up to two weeks in the case of fungal infections.17 Antimicrobial sensitivity reports may also be obtained from the laboratory.17 Once a culture has been performed and sent out, immediately begin empiric antimicrobial therapy based on clinical signs and history, if you haven’t already.6,17 In the case of a negative culture, cessation of antibiotic therapy for 12 to 24 hours may be considered to increase pathogen yield.11
Treatment of Corneal Ulcers
When initiating treatment of keratitis, corneal infiltrates must first be classified as sterile or infectious in nature.18
Sterile keratitis. These infiltrates are described as minor defects, usually less than 1mm to 1.5mm in size, and are associated with negligible pain, discharge, conjunctival inflammation and anterior chamber reaction, as well as with absent or minimal epithelial involvement.18,19 The majority of sterile infiltrates are subepithelial or anterior stromal and are found in the mid-peripheral to peripheral cornea within approximately 4mm from the limbus.18,19 Sterile infiltrates may be associated with contact lens wear, particularly in cases of extended wear and poor hygiene, and tend to occur in the superior corneal quadrant in the setting of extended contact lens wear.18,19
Initial treatment options for presumed sterile infiltrates include discontinuation of lens wear followed by topical antibiotics, fortified topical antibiotics, antibiotic- steroid combo agents, topical steroids alone or simply observation. Microbial culture is not indicated in cases of sterile infiltrates.18 Topical antibiotic-steroid combinations have been shown to resolve sterile infiltrates in less time than topical antibiotics alone.19 If a distinct epithelial defect develops along with pain and ocular inflammation, a bacterial ulcer may be underlying and requires prompt treatment with antibiotic therapy, with initial avoidance of steroid use.18,19
Infectious keratitis. When you see stromal infiltrates in the presence of epithelial disruption, particularly in cases of bacterial disease, you’re quite likely dealing with an infectious etiology.4,5,7 Patients with infectious corneal ulcers are typically acutely symptomatic for pain, photophobia, decreased vision, conjunctival injection and mucopurulent discharge.4,7,11 Severe cases may result in stromal thinning, ulceration, corneal perforation, endophthalmitis, vision loss secondary to stromal scarring or even loss of the eye.3,5,8,11
Prompt empiric treatment is generally necessary when culture and/or sensitivity testing is unavailable or hasn’t yet been performed.3,11 The regimen should consist of topical broad-spectrum antibiotics, such as fluoroquinolones or fortified aminoglycosides, with a cephalosporin or vancomycin.7 Consider geography, and local bacterial prevalence and antibiotic sensitivity, when selecting the antibiotic of choice.3,5
While a large number of clinical trials have been published, there is currently no unanimous treatment regimen detailing which antibiotics should be used for bacterial keratitis.3 Numerous sample regimens have been detailed, and are subdivided based on microorganism characteristics (see Table 1).3 Presently, ciprofloxacin 0.3%, levofloxacin 1.5% and ofloxacin 0.3% are FDA-approved to treat bacterial keratitis.11
In cases of large or sight-threatening ulcers, particularly if accompanied by a hypopyon or deep stromal involvement, fortified topical antibiotics are beneficial, and a loading dose every five to 15 minutes followed by hourly application is recommended.11 Subconjunctival injection, systemic therapy or hospitalization may be necessary in severe cases.3,11 Adjuctive cycloplegics also may be considered in instances of significant anterior chamber reaction and/or severe pain due to their palliative and anti-inflammatory properties.1
Steriod Therapy
While topical antibiotic therapy remains the mainstay treatment of bacterial keratitis, adjunctive corticosteroid use may also prove beneficial for clinical outcomes, although use of topical steroids for microbial keratitis remains controversial.4,5,8 Proponents contend that steroids mitigate tissue damage from the host inflammatory response by decreasing severity of stromal melting, neovascularization and scarring.4,5,8 Furthermore, steroids may improve patient compliance with antibiotic treatment by alleviating pain and discomfort.5 Conversely, steroid therapy may delay epithelial healing and potentiate bacterial keratitis, leading to stromal thinning and melting.4,5,20
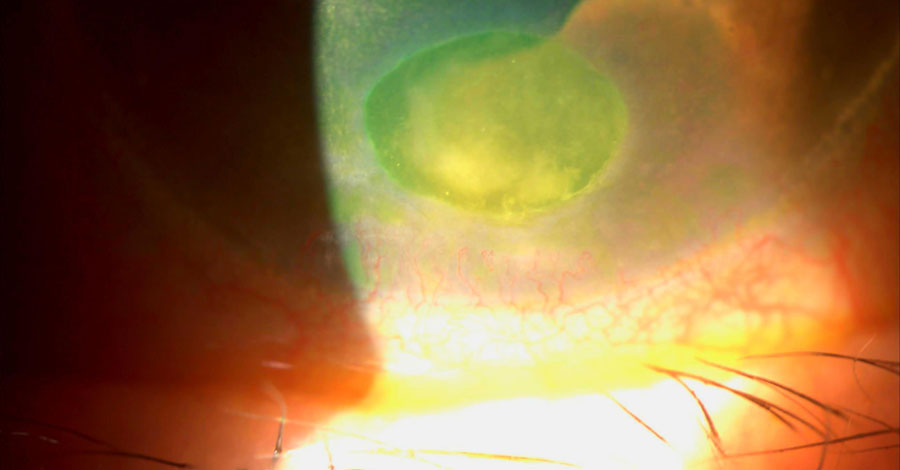 |
This patient had ruptured bullae and subsequently developed a bacterial ulcer. Click image to enlarge. |
Four clinical trials, including one randomized, placebo-controlled, double-masked trial known as SCUT, have compared clinical outcomes in bacterial keratitis treated with antibiotics and steroids vs. antibiotics alone.5,21 While the first three smaller trials evidenced neither harm nor benefit associated with topical steroid use, subgroup analysis within SCUT found that patients with low vision, or deep or central ulcers, at baseline experienced better visual improvement at three months when compared with placebo, as did patients with invasive Pseudomonas strains.5,21 No significant difference in adverse effects was noted between steroid and placebo arms.5,20,21
Timing and dosage are important concerns, as patients who began steroids after only two to three days of antibiotic use, particularly with potent agents administered six times daily, experienced better visual outcomes than those in the placebo arm. Patients with later steroid use experienced neutral or worse acuity vs. placebo.5,20-22
A 12-month follow-up of the SCUT trial also demonstrated superior visual outcomes in patients with ulcers not caused by Nocardia keratitis (NK).23 Patients with NK, however, have been found to experience larger infiltrates and scars with corticosteroid therapy, despite no difference in vision, re-epithelialization or corneal perforation. Poor outcomes with steroid use have also been associated with fungal and Acanthamoeba infections.22,24
To minimize adverse effects, it is prudent to administer topical steroids after 24 to 48 hours of antibiotic therapy, following evidence of ulcer improvement and microorganism identification via culture.4,5,11 Avoidance of topical steroids in cases of atypical keratitis, as well as in NK, fungal and Acanthamoeba etiologies, is critical.5,11
Adjuvant Therapy
In addition to traditional antibiotic therapy, several adjuvant options for bacterial keratitis have been described. A randomized, controlled trial demonstrated that low-concentration topical povidone-iodine 1.25% may perform with equal efficacy as topical antibiotics in the treatment of bacterial keratitis, and has the added benefit of considerably lower cost.25 Collagen crosslinking, a procedure which treats corneal ectatic conditions by strengthening stromal tissue, has been shown to be of potential benefit for recalcitrant infectious keratitis by halting corneal melting, resolving ulcers resistant to treatment and improving patient symptoms.8
The use of tetracyclines such as doxycycline has been suggested due to their antimetalloproteinase properties, which may lessen the risk of severe complications of infectious keratitis, such as corneal perforation.8 Other adjunctive therapies proposed for bacterial keratitis include amniotic membranes, antibiotic-soaked soft contact lenses, collagen shields, mitomycin-C, hyperbaric oxygen therapy, autologous serum eye drops and cryotherapy, although the beneficial effects of these therapies are not universally established.11,26
When to Change Therapy
Monitoring clinical response to treatment is critical to success—we need to establish if the therapy is working and whether modification is necessary.8,11 Lack of clinical response or worsening of signs and symptoms within 48 hours, particularly in cases of vision-threatening ulcers, may warrant initiating a more aggressive antibiotic regimen (e.g., switching to fortified broad-spectrum antibiotics if initial treatment consisted of fluoroquinolone therapy), considering less common microorganisms, performing a re-culture or referring to a cornea specialist.7,8,11,27
In cases of unlikely compliance, disease extension into adjacent tissues or threat of corneal perforation, systemic antibiotics or hospitalization may be necessary.11 Also consider that drug toxicity may be mistaken for lack of clinical improvement, and reduction of therapy may be warranted at times in order to assess healing.8,11
Bacterial keratitis remains a prominent cause of vision loss worldwide. Precise clinical identification of underlying etiology is critical for effective treatment, and may be aided by proper microbial culture. Although antibiotic therapy remains first-line for bacterial ulcers, adjuvant treatments have been described, and corticosteroid therapy may play an essential role in improved visual outcomes.
Dr. Sherman is an assistant professor of optometric sciences (in ophthalmology) and the director of optometry at Columbia University Medical Center. She specializes in complex and medically necessary contact lens fittings and ocular disease. She is a fellow of the American Academy of Optometry. Ms. Cherny is a 2021 optomery candidate at SUNY and will be completing her ocular disease/contact lens residency at Mass Eye and Ear. They have no financial interests to disclose.
1. Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87(7):834-838. 2. Ballouz D, Maganti N, Tuohy M, Errickson J, Woodward MA. Medication burden for patients with bacterial keratitis. Cornea. 2019;38(8):933-937. 3. McDonald EM, Ram FS, Patel DV, McGhee CN. Topical antibiotics for the management of bacterial keratitis: an evidence-based review of high quality randomised controlled trials. Br J Ophthalmol. 2014;98(11):1470-7. 4. Ni N, Srinivasan M, McLeod SD, Acharya NR, Lietman TM, Rose-Nussbaumer J. Use of adjunctive topical corticosteroids in bacterial keratitis. Curr Opin Ophthalmol. 2016;27(4):353-357. 5. Palioura S, Henry CR, Amescua G, Alfonso EC. Role of steroids in the treatment of bacterial keratitis. Clin Ophthalmol. 2016;10:179-186 6. Dalmon C, Porco TC, Lietman TM, et al. The clinical differentiation of bacterial and fungal keratitis: a photographic survey. Invest Ophthalmol Vis Sci. 2012;53(4):1787-1791. 7. Moshirfar M, Hopping GC, Vaidyanathan U, Liu H, Somani AN, Ronquillo YC, Hoopes PC. Biological staining and culturing in infectious keratitis: controversy in clinical utility. Med Hypothesis Discov Innov Ophthalmol. 2019;8(3):145-151. 8. Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124(11):1678-1689. 9. Mascarenhas J, Lalitha P, Prajna NV, Srinivasan M, Das M, D’Silva SS, Oldenburg CE, Borkar DS, Esterberg EJ, Lietman TM, Keenan JD. Acanthamoeba, fungal, and bacterial keratitis: a comparison of risk factors and clinical features. Am J Ophthalmol. 2014;157(1):56-62. 10. Thomas PA, Leck AK, Myatt M. Characteristic clinical features as an aid to the diagnosis of suppurative keratitis caused by filamentous fungi. Br J Ophthalmol. 2005;89(12):1554-8. 11. Lin A, Rhee MK, Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, Varu DM, Musch DC, Dunn SP, Mah FS. Bacterial keratitis preferred practice pattern. Ophthalmology. 2019;126(1):P1-P55. 12. Dahlgren MA, Lingappan A, Wilhelmus KR. The clinical diagnosis of microbial keratitis. Am J Ophthalmol. 2007;143(6):940-944. 13. Levey SB, Katz HR, Abrams DA, Hirschbein MJ, Marsh MJ. The role of cultures in the management of ulcerative keratitis. Cornea.1997;16(4):383-6. 14. Gupta N, Tandon R. Investigative modalities in infectious keratitis. Indian J Ophthalmol. 2008;56(3):209-213. 15. Pakzad-Vaezi K, Levasseur SD, Schendel S, Mark S, Mathias R, Roscoe D, Holland SP. The corneal ulcer one-touch study: a simplified microbiological specimen collection method. Am J Ophthalmol. 2015;159(1):37-43. 16. McLeod SD, Kolahdouz-Isfahani A, Rostamian K, Flowers CW, Lee PP, McDonnell PJ. The role of smears, cultures, and antibiotic sensitivity testing in the management of suspected infectious keratitis. Ophthalmology. 1996;103(1):23-8. 17. Robinson J, Ellen J, Hadel B, Lighthizer N. Collecting a corneal culture. Rev Optometry. 2016; 153(4):64-71. 18. Baum J, Dabezies OH. Pathogenesis and treatment of “sterile” midperipheral corneal infiltrates associated with soft contact lens use. Cornea. 2000;19(6):777-781. 19. Donshik PC, Suchecki JK, Ehlers WH. Peripheral corneal infiltrates associated with contact lens wear. Trans Am Ophthalmol Soc. 1995;93:49-64. 20. Green M, Hughes I, Hogden J, Sara S, Apel A, Stapleton F. High-dose steroid treatment of bacterial keratitis. Cornea. 2019;38(2):135–140. 21. Srinivasan M, Mascarenhas J, Rajaraman R, et al. Steroids for Corneal Ulcers Trial Group. Corticosteroids for bacterial keratitis: the steroids for corneal ulcers trial (SCUT). Arch Ophthalmol. 2012 Feb;130(2):143-50. 22. Ray KJ, Srinivasan M, Mascarenhas J, Rajaraman R, Ravindran M, Glidden DV, Oldenburg CE, Sun CQ, Zegans ME, McLeod SD, Acharya NR, Lietman TM. Early addition of topical corticosteroids in the treatment of bacterial keratitis. JAMA Ophthalmol. 2014;132(6):737-41. 23. Srinivasan M, Mascarenhas J, Rajaraman R, et al. The steroids for corneal ulcers trial (SCUT): secondary 12-month clinical outcomes of a randomized controlled trial. Am J Ophthalmol. 2014;157(2):327-333. 24. Lalitha P, Srinivasan M, Rajaraman R, et al. Nocardia keratitis: clinical course and effect of corticosteroids. Am J Ophthalmol. 2012 Dec;154(6):934-939 25. Isenberg SJ, Apt L, Valenton M, Sharma S, Garg P, Thomas PA, Parmar P, Kaliamurthy J, Reyes JM, Ong D, Christenson PD, Del Signore M, Holland GN. Prospective, Randomized Clinical Trial of Povidone-Iodine 1.25% Solution Versus Topical Antibiotics for Treatment of Bacterial Keratitis. Am J Ophthalmol. 2017 Apr;176:244-253. 26. Dakhil TAB, Stone DU, Gritz DC. Adjunctive therapies for bacterial keratitis. Middle East Afr J Ophthalmol. 2017;24(1):11-17. 27. Weiner, Gabrielle. Confronting Corneal Ulcers. American Academy of Ophthalmology, 2012. www.aao.org/eyenet/article/confronting-corneal-ulcers. |

