What's Powering the Next Wave?In the September issue—our 45th annual technology report—find out how AI, telehealth, OCT and other new clinical devices are helping ODs build out their practice for the future. Check out the other featured articles here:
|
There are 37.5 million diabetic Americans, and less than half have historically received dilated diabetic eye exams at recommended intervals, leaving the majority without proper care and surveillance.1-7 Although there are many ideas for how to fill this void, one promising and emerging solution to increase access to diabetic retinopathy (DR) screening is artificial intelligence (AI).6-8 Will such systems replace optometrists and ophthalmologists or merely augment our capabilities? At which level of care are they the most useful? Let’s look at where things currently stand.
Background
AI is defined by the National Artificial Intelligence Act of 2020 as “a machine-based system that can for a given set of human-defined objectives, make predictions, recommendations or decisions influencing real or virtual environments.”9 Machine learning is a subset of AI that designs and trains software algorithms to learn from and then make predictions, recommendations or decisions on data. Machine learning can incorporate artificial neural networks. In this type of system, a digital input, such as a retinal image, is presented to the first layer of neurons, or nodes. The input in each layer of nodes is processed and converted into outputs, which are then transmitted to the next layer for further processing.
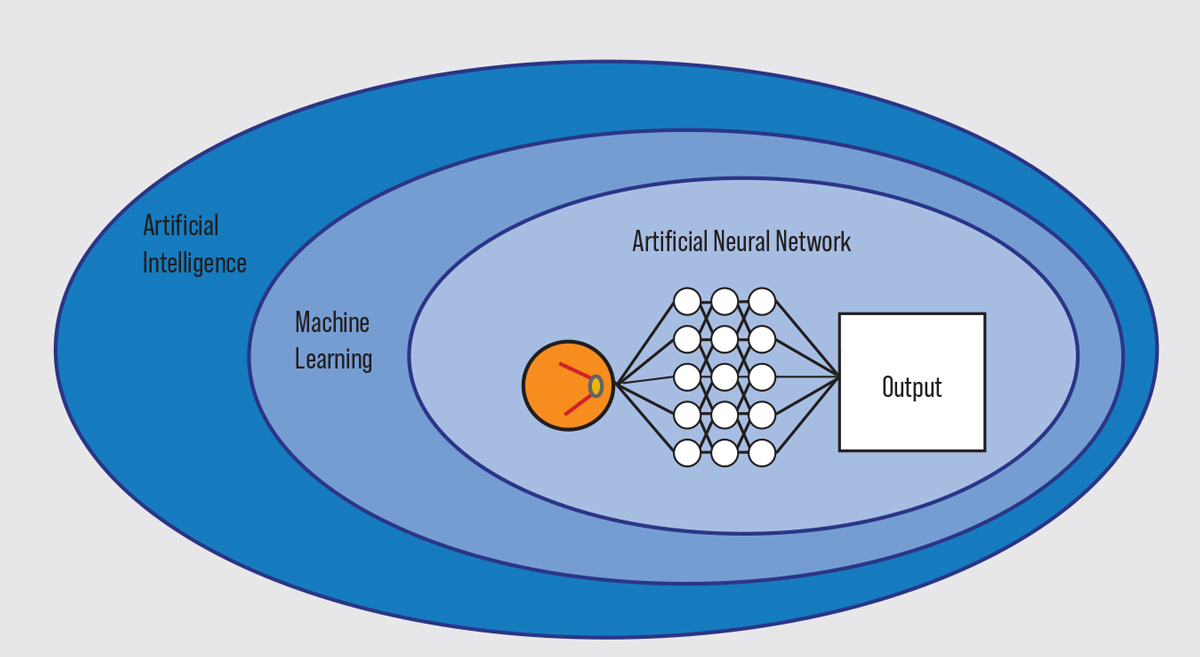 |
| A depiction of essential AI vocabulary. Click image to enlarge. |
During the algorithm training, the final output is compared with a reference standard to determine if further modification and training is required. After training, the algorithm is validated with another dataset to verify its ability to produce an accurate outcome.10 Machine learning algorithms differ in the production time, size of training data, learning method and structure of the artificial neural network and goals of the final output resulting in a wide variety of potential designs.11
AI Today
When deployed clinically, these algorithms can be applied as assistive AI or autonomous AI. Assistive AI can be used in conjunction with a provider to aid in diagnosis and disease severity grading. Autonomous AI works independently of providers to diagnose disease. To date, autonomous AI has two primary ways of detecting DR: detector-based design and deep learning. As with clinicians, detector-based designs identify abnormalities based on various biomarkers indicative of DR, including microaneurysms, hemorrhages, venous anomalies, exudates and neovascularization. Deep learning is often referred to as a black box system because it is less clear how the algorithm learns to identify disease.13
There are two AI devices for DR currently available for clinical use in the United States: IDx-Dr by Digital Diagnostics and EyeArt by Eyenuk. Both technologies have been evaluated for their ability to detect DR by quantifying imageability, sensitivity and specificity of their performance and comparing them with human-interpreted images.13
 |
| The IDx-DR could be a reliable screening tool that may uncover diabetic eye disease in the primary care office. Photo: Digital Diagnostics. Click image to enlarge. |
IDx-DR (Digital Diagnostics). This instrument was FDA de novo cleared in 2018 as the first autonomous AI system in medicine.14 In the IDx-DR pivotal trial, among 900 patients in primary care clinics were evaluated for more-than-mild DR and/or center-involved diabetic macular edema (DME). It had a sensitivity of 87.2% and a specificity of 90.7% when compared with the Wisconsin Fundus Photograph Reading Center analysis of four widefield stereoscopic dilated fundus photographs, anterior segment photography and a macular optical coherence tomography (OCT) scan. There was an imageability rate of 96.1%, with 23.6% of patients requiring dilation.15 IDx-DR has been installed in hundreds of point-of-care clinics, including primary care physicians, endocrinology, clinical laboratories and retail clinics, with the goal of early detection and intervention of diabetic eye disease.
In practice, a primary care physician orders testing for patients who are 22 and older, have not been previously diagnosed with DR and are not otherwise contraindicated. Two non-mydriatic retinal images of each eye, one disc-centered and one macula-centered, are captured by an operator with guidance from the system and uploaded to a secure cloud-based system. Using a lesion detection algorithm, the device analyzes the images using biomarker-based AI to detect more-than-mild DR and center-involved DME.
The findings and care recommendations are provided on a one-page report that includes a diagnostic output that aligns with the American Academy of Ophthalmology’s Preferred Practice Pattern recommendations. Patients with more-than-mild DR and/or DME are referred to optometrists and ophthalmologists for evaluation and proper management; otherwise, IDx-DR generates a recommendation of continued yearly examination.16
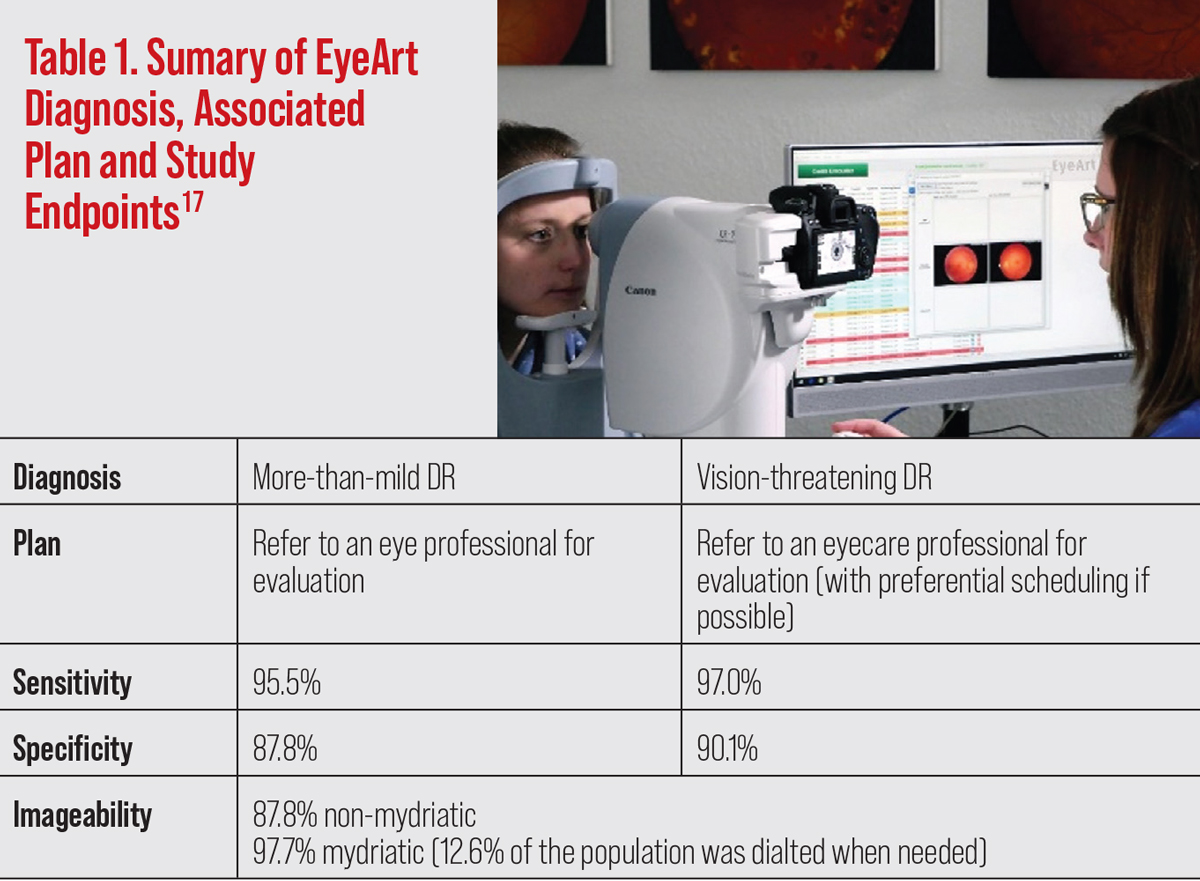
EyeArt AI Eye Screening System (Eyenuk). The manufacturer received 510(k) clearance by the FDA to market the EyeArt AI system for the detection of more-than-mild DR and vision-threatening DR in August 2020.17 It has many similarities to the IDx-DR.
EyeArt was validated in a prospective, multicenter pivotal clinical trial with 942 participants that demonstrated the system’s ability for detecting more-than-mild DR (sensitivity 95.5% and specificity 87.8%) and vision-threatening DR (sensitivity 97.0% and specificity 90.1%). This too was compared with the Wisconsin Fundus Photograph Reading Center analysis of four widefield stereoscopic dilated fundus photographs and anterior segment photography. Imageability was reported at 87.6% in undilated funduscopic images. In patients who required dilation, the imageability rate was 97.7%.18
Much like the IDx-DR, the EyeArt is indicated to evaluate individuals 22 and older who have diabetes and have not been previously diagnosed with more-than-mild DR or other contraindications. Eyenuk is working with a few hundred installations deployed in testing locations, including hospitals, primary care offices, independent diabetic clinics, insurance companies, ophthalmology practices and optometry practices.
After obtaining a disc-centered and a macula-centered photo of each eye, the system generates a report of the diagnosed level of disease and an associated plan, also based on the Academy of Ophthalmology’s Preferred Practice Pattern recommendations.
Eyenuk also offers diagnostic services in an asynchronous telehealth setting that combines human readers and AI assessment to detect DR and other ocular diseases.
The OD’s Role Today
Optometrists have a responsibility to understand the capability, benefits and limitations of AI algorithms in diabetic eye care. Al can democratize fundus photo interpretation by leveling the playing field for providers with varying clinical expertise. Increasing access to fundus photography and interpretation also increases access to DR screenings and opportunities for early interventions, especially for patients not currently managed by an eyecare professional. AI does not threaten to replace optometrists; it is a tool to improve patient access and equity.
AI is an emerging technology that is intended to supplement eye care rather than replace a comprehensive eye exam. AI-based DR screenings strive to ensure that the patients most in need—those with more-than-mild DR—have access to care. It does not account for patients who have other retinal diseases, optic nerve disease or ocular comorbidities associated with fluctuating glycemia (e.g. diabetic cataracts and neurotrophic keratitis).
Like with most new technologies, there is a level of uncertainty regarding the potential implications and its effect on the future of healthcare. To ensure responsible and efficient implementation of AI for DR detection, conversation should center around current areas of uncertainty: the doctor-patient relationship, referrals and continuum of care, high patient demand and the long-term metrics to indicate positive patient outcomes. These uncertainties are likely to be resolved over time but are a limiting factor in the current confidence of AI. Although there are some clear limitations in the technology today, AI is a tool that could potentially change recommended exam intervals and other standards of care for diabetic examinations in the future.
AI for DR detection is being implemented in two clinical models: as a screening for healthcare and as an additional clinical tool in eyecare facilities.
One strategy to increase retinal screening is to incorporate it into a point-of-care visit during routinely scheduled diabetic care. Hemoglobin A1c measurements are a screening tool with 60% to 90% adherence among diabetic patients.3,19 This makes clinics that perform A1c measurements—primary care, endocrinology and clinical laboratories—ideal places to incorporate diabetic eye screening.
Furthermore, those practitioners are incentivized to participate in diabetic eye screenings with the help of clinical quality measurement programs including the Centers for Medicare and Medicaid Services’ Merit-Based Incentive Payment System and the National Committee for Quality Assurance’s Healthcare Effectiveness Data and Information Set (HEDIS). These quality metrics can be met at the point-of-care using autonomous AI systems that provide quick reports containing findings and recommendations at the point-of-care. This point-of-care model focuses on identifying patients who do not obtain routine diabetic eye exams to help ensure they receive the eye care required to prevent diabetes-related vision loss.
Point-of-care DR screening will likely increase DR identification and require those patients to seek care with an eyecare professional. Optometrists are essential in this model to build relationships with providers in these other areas of diabetic care, to evaluate the patients flagged at point-of-care and to help medically manage these patients.
EyeArt is used in eyecare settings, where it has been deployed as a screening tool and a confirmation for human diagnosis. AI as a screening tool can be used in high volume clinics or during community screening to allow practitioners to prioritize those with more immediate needs.
It can also be used as a tool for confirmation of DR staging and a method of communicating to retinal specialists. The application of current AI technologies for DR has the potential to increase consistency of diagnosis.20 AI can also streamline clinical care and add tools for better delivery of diabetic eye care.
 |
| Click image to enlarge. |
AI Tomorrow
This technology will undoubtedly continue to evolve and expand throughout health care and eye care. Looking at the next three to five years, new and upcoming AI technologies can be divided into the hardware in which the algorithm will be incorporated: fundus cameras and OCTs.
Fundus cameras. There are dozens of companies designing algorithms that use fundus photography for lesion detection for DR. During the design phase of a multicenter, head-to-head, real-world validation study of seven Automated AI DR screening systems, published in Diabetes Care, 23 companies with AI algorithms were invited to participate.21 The following list of AI algorithms for DR are the ones that were announced to be in FDA trials in 2022 and moving towards FDA clearance:
• AEye (AEye Health) and Aurora (Optomed) are working in collaboration for autonomous detection of more-than-mild DR. In a 2022 press release AEye Health announced the results of the pivotal FDA clinical trial.22 Using a single photo from a Topcon NW-400 as reference, the system achieved 93% sensitivity, 91.4% specificity and >99% imageability. Using a single photo from a handheld camera (Optomed Aurora), 91.9% sensitivity, 93.6% specificity and >99% imageability was achieved. If FDA cleared, this technology could contribute an AI algorithm that requires a single photo and an algorithm built to work with a handheld fundus camera.
Looking towards the future, AEye released a preprint paper in July 2022 that outlines the potential use of AI to predict future development of DR, which could influence personalized patient prognosis and management.23
• Automated Retinal Disease Assessment (ARDA; Verily Life Sciences) is an algorithm currently used in India and across the European Union.24 Verily Life Sciences and Google are analyzing the ARDA algorithm for more-than-mild DR on both 45º and ultra-widefield fundus imaging.25 No results have yet been released. If this receives FDA clearance, ARDA could be the first AI algorithm for DR detection in ultra-widefield fundus imaging.
• A company called Retina-AI is developing a DR algorithm known as Galaxy that can work on several different imaging devices. The Retina-AI recently released a study that compared five different fundus cameras in detecting more-than-mild DR and vision-threatening DR. According to company data, there is at least 77.4% sensitivity, 82.4% specificity and 94% imageability between cameras and variable levels of disease.26,27 Retina-AI initiated another FDA trial in May 2022 focusing on DR detection in three retinal cameras.28 If FDA cleared, this algorithm could increase availability of AI-powered software across various fundus cameras.
• Zilia Ocular (Zilia) is a hybrid retinal camera that can also incorporate oximetry, a measurement of blood oxygen saturation. The camera has a 24º field of view with fixation targets that allow imaging throughout the retina. The company is investigating the use of computerized neural networks to improve oximetry calculations.29 In parallel with imaging, machine learning algorithms will be used to quantify oxygen saturation along with other biomarkers. This will help identify early spectral and structural changes for a variety of diseases, including diabetes and DR. Zilia is aiming to receive FDA clearance by 2024.
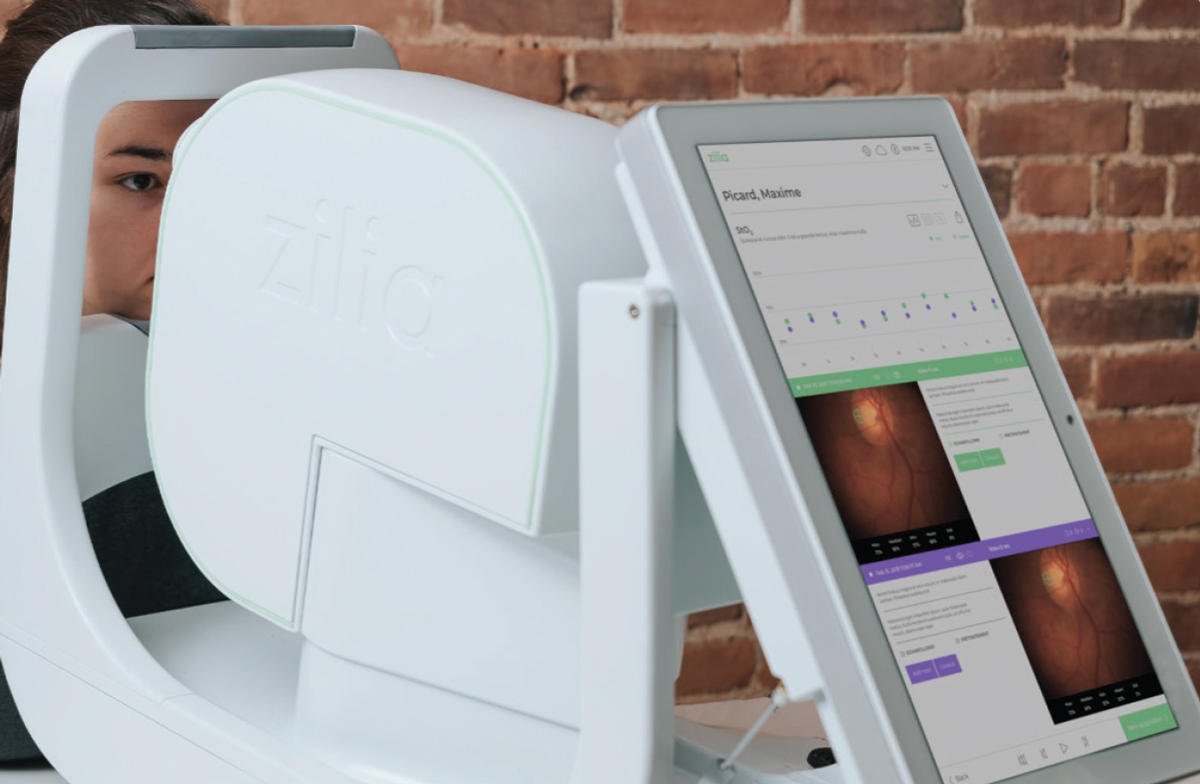 |
| Zilia has a hybrid retinal camera in development that can potentially incorporate oximetry, a measurement of blood oxygen saturation. Photo: Zilia. Click image to enlarge. |
OCT. Currently, FDA-cleared applications of AI algorithms in OCTs are limited to age-related macular degeneration (AMD). However, active research on integrating AI into OCTs for DME detection is likely to impact clinical care. Detecting DME with funduscopic examination and fundus photography is well documented to result in low sensitivity, making the OCT a crucial tool in preventative care here.15,30-33
Integrating AI into this tool can democratize and streamline diabetic eye care. Early research and current clinical applications indicate that AI embedded in the OCT will likely serve as another resource for improving detection and management protocols of DME.
The Notal OCT Analyzer (Notal Vision) is a leader in applications of AI in OCTs. The algorithm was granted FDA breakthrough device designation in 2018 for patients with wet AMD.34 It was recently featured in the AREDS2 10-year follow-up study, which compared intraretinal and subretinal fluid detection between the Notal OCT Analyzer and human investigators.
Upon comparing intraretinal fluid detection, a relevant biomarker in DME, the Notal OCT Analyzer was superior in accuracy (0.877 vs. 0.815) and sensitivity (0.763 vs. 0.403), while investigators were superior in specificity (0.922 vs. 0.978) and precision (0.795 vs. 0. 879). The study concluded that this device and equivalent tools may have a future in clinical care for detecting retinal fluid to improve specificity and ultimately better diagnose and manage patients as well as determine prognoses.35
As these technologies continue to mature, it is likely that intraretinal fluid detection from OCT will expand to help in the detection and management of other diseases, including DME. Early detection can allow for early intervention and avoid cases of preventable blindness associated with DME.
Takeaways
Today, autonomous AI is being incorporated into fundus photography to detect DR. It is used to primarily screen patients at the point-of-care to reduce gaps in care and thereby improve health equity. Simultaneously, it has already begun to assist eyecare providers within their clinics. Optometrists should understand the technology’s design and real-world validation results to fully evaluate its strengths and weaknesses and understand how it can be implemented appropriately in different clinical care settings.
During this digital revolution, AI is expected to continually become more prominent throughout health care. As it relates to eye care for those with diabetes, autonomous algorithms are expected to further infiltrate retinal cameras and OCT markets. Optometrists will continue to have an increased responsibility to decide how to best incorporate this emerging technology into their practices and patient care.
1. Benoit SR, Swenor B, Geiss LS, et al. Eye care utilization among insured people with diabetes in the US, 2010–2014. Diabetes Care. 2019;42(3):427-33. 2. Centers for Disease Control and Prevention. National diabetes statistics report. January 18, 2022. www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed July 15, 2022. 3. National Committee for Quality Assurance. Comprehensive diabetes care (CDC). NCQA. www.ncqa.org/hedis/measures/comprehensive-diabetes-care/. Accessed July 15, 2022. 4. Shi Q, Zhao Y, Fonseca V, et al. Racial disparity of eye examinations among the US working-age population with diabetes: 2002-2009. Diabetes Care. 2014;37(5):1321-8. 5. Fathy C, Patel S, Sternberg P, Kohanim S. Disparities in adherence to screening guidelines for diabetic retinopathy in the United States: a comprehensive review and guide for future directions. Semin Ophthalmol. 2016;31(4):364-77. 6. Lundeen EA, Wittenborn J, Benoit SR, Saaddine J. Disparities in receipt of eye exams among Medicare Part B fee-for-service beneficiaries with diabetes —United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68(45):1020-3. 7. Gibson DM. Estimates of the percentage of US adults with diabetes who could be screened for diabetic retinopathy in primary care settings. JAMA Ophthalmol. 2019;137(4):440-4. 8. Fathy C, Patel S, Sternberg P, Kohanim S. Disparities in adherence to screening guidelines for diabetic retinopathy in the United States: a comprehensive review and guide for future directions. Semin Ophthalmol. 2016;31(4):364-77. 9. US Food and Drug Administration. artificial intelligence and machine learning in software as a medical device. September 22, 2021. www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-software-medical-device#whatis. Accessed July 15, 2022. 10. Tsiknakis N, Theodoropoulos D, Manikis G, et al. Deep learning for diabetic retinopathy detection and classification based on fundus images: a review. Computers in Biology and Medicine. 2021;135:104599. 11.Vizient. TechFlash: artificial intelligence for automated detection of diabetic retinopathy. 2021. www.vizientinc.com/-/media/documents/sitecorepublishingdocuments/secured/collaboratives/techflash_ai_diabetic_retinopathy_aug_2021.pdf?RIN2104. Accessed July 15, 2022. 12. Channa R, Wolf R, Abramoff MD. Autonomous artificial intelligence in diabetic retinopathy: from algorithm to clinical application. J Diabetes Sci Technol. 2021;15(3):695-8. 13. Schmidt-Erfurth U, Sadeghipour A, Gerendas BS, et al. Artificial intelligence in retina. Porg Retin Eye Res. 2018;67:1-29. 14. Chiang MF. artificial intelligence getting smarter! innovations from the vision field. National Eye Institute. February 8, 2022. directorsblog.nih.gov/tag/idx-dr/. Accessed July 15, 2022. 15. Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. npj Digit Med. 2018;1(1):39. 16. US Food and Drug Administration, Center for Drug Evaluation and Research. IDx-DR de novo summary (DEN180001). January 12, 2018. www.accessdata.fda.gov/cdrh_docs/reviews/DEN180001.pdf. Accessed July 15, 2022. 17. US Food and Drug Administration, Center for Drug Evaluation and Research. EyeArt 501(k) summary (K200667). August 3, 2020. www.accessdata.fda.gov/cdrh_docs/pdf20/K200667.pdf. Accessed July 15, 2022. 18. Ipp E, Liljenquist D, Bode B, et al. Pivotal Evaluation of an artificial intelligence system for autonomous detection of referrable and vision-threatening diabetic retinopathy. JAMA Netw Open. 2021;4(11):e2134254. 19. Baughman D, Zain A, Waheed A. Patient adherence to hemoglobin a1c testing recommendations in telemedicine and in-office cohorts during COVID-19. JAMA Netw Open. 2021;4(9):e2127779. 20. Lin DY, Blumenkranz MS, Brothers RJ, Grosvenor DM. The sensitivity and specificity of single-field nonmydriatic monochromatic digital fundus photography with remote image interpretation for diabetic retinopathy screening: a comparison with ophthalmoscopy and standardized mydriatic color photography. Am J Ophthalmol. 2002;134(2):204-13. 21. Lee AY, Yanagihara RT, Lee CS, et al. Multicenter, head-to-head, real-world validation study of seven automated artificial intelligence diabetic retinopathy screening systems. Diabetes Care. 2021;44(5):1168-75. 22. James M. AEYE Health Reports Pivotal Clinical Trial Results of its AI Algorithm for the Autonomous Screening and Detection of More-Than-Mild Diabetic Retinopathy. PR Newswire. February 7, 2022. www.prnewswire.com/news-releases/aeye-health-reports-pivotal-clinical-trial-results-of-its-ai-algorithm-for-the-autonomous-screening-and-detection-of-more-than-mild-diabetic-retinopathy-301476299.html. Accessed July 15, 2022. 23. Rom Y, Aviv R, Ianchulev S, Dvey-Aharon Z. Predicting the future development of diabetic retinopathy using a deep learning algorithm for the analysis of non-invasive retinal imaging. medRxiv. January 1, 2022. [Epub ahead of print]. 24. Google Health. Using AI to prevent blindness. health.google.com/caregivers/arda/. Accessed July 15, 2022. 25. Verily Life Sciences. ARDA Software for the Detection of mtmDR. June 6, 2022. clinicaltrials.gov/ct2/show/NCT04905459. Accessed July 15, 2022. 26. Odaibo S, Imasogie, N, Courtes A, et al. Pivotal study of an autonomous multi-camera compatible artificial intelligence system for diabetic retinopathy screening in primary care. Presented at SIIM Annual Meeting 2021. 27. RETINA-AI Health. Validation study of RETINA-AI Galaxy, an automated diabetic retinopathy screening device. clinicaltrials.gov/ct2/show/NCT04774822. Accessed July 15, 2022. 28. RETINA-AI Health. Validation study of RETINA-AI Galaxy v2.0, an automated diabetic retinopathy screening device. clinicaltrials.gov/ct2/show/NCT05368623. Accessed July 15, 2022. 29. DePaoli DT, Tossou P, Parent M, et al. Convolutional neural networks for spectroscopic analysis in retinal oximetry. Sci Rep. 2019;9(1):11387. 30. Ferris FL 3rd, Patz A. Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol. 1984;28 Suppl:452-61. 31. Centers for Disease Control and Prevention. Diabetes and vision loss. Published online May 7, 2021. www.cdc.gov/diabetes/managing/diabetes-vision-loss.html. Accessed July 15, 2022. 32. Browning DJ, McOwen MD, Bowen RMJ, O’Marah TL. Comparison of the clinical diagnosis of diabetic macular edema with diagnosis by optical coherence tomography. Ophthalmology. 2004;111(4):712-5. 33. Wang YT, Tadarati M, Wolfson Y, et al. Comparison of prevalence of diabetic macular edema based on monocular fundus photography vs. optical coherence tomography. JAMA Ophthalmology. 2016;134(2):222-8. 34. Hutton D. Efficacy of Notal Vision OCT device demonstrated by a series of scientific and clinical work. Ophthalmology Times. July 1, 2022. www.ophthalmologytimes.com/view/efficacy-of-notal-vision-oct-device-demonstrated-by-a-series-of-scientific-and-clinical-work. Accessed July 15, 2022. 35. Keenan TDL, Clemons TE, Domalpally A, et al. Retinal specialist vs. artificial intelligence detection of retinal fluid from OCT. Ophthalmology. 2021;128(1):100-9. |


