IT WAS NOT LONG AGO that high technology for retinal imaging in the optometric office consisted of a non-mydriatic Polaroid camera. Although these cameras do a fine job of photodocumenting the posterior pole, they do not capture the peripheral retina, produce images that can be manipulated, or capture any sense of a cross-sectional view of either the optic nerve or macula. Older technologies do a fine job of monitoring for incremental change over time, but they cannot compare to the technologies mentioned in this article.
When it comes to retinal imaging, today’s technologies make the possibilities endless. Digital images are capable of being zoomed, enhanced and changed in other ways for both the central pole and the peripheral retina. Retinal cross-sectional images can detect changes so small that it is hard to fathom. It is now possible to take measurements of the macula to proactively determine risk for macular degeneration. If patients develop AMD, we can now monitor for the earliest stages of CNVM and allow for more effective treatment. Scanning laser systems can monitor for macular edema without the need for introduction of fluorescein into the body. Beyond the macula, the optic nerve and nerve fiber layer can be closely monitored for subtle changes that may be the first signs of glaucoma.
Not only are all of these things possible, they are practical. This article reviews how these technologies work and where they fit into our current practices. Additionally, it will help us envision our practices of the future, if these technologies are not already in place. We will also discuss the importance of good clinical skills and judgment, as none of the instruments mentioned will replace the need for well-trained, experienced clinicians.
Practice setting and environment are key factors in determining the need for new retinal technology. For this reason, three esteemed colleagues from three different practice environments have contributed their perspectives to this article. What makes sense for one O.D. or practice may not pertain to another. With the views of these three forward-thinking and cutting- edge doctors, we can see first-hand how to incorporate technology into our practices and ultimately benefit our patients. From “low tech” nutritional supplements to “high tech” instruments, this article will discuss how to successfully incorporate technology into your particular practice setting.
Utilizing Retinal Technology for Prevention/Detection of Macular Disease
By Pamela A. Lowe, O.D., F.A.A.O.
As primary eye care practitioners, our first and foremost goal is the preservation of our patient’s precious sense of sight. To this end, our profession has the great privilege of being on the front line for educating the public on conditions that can rob them of a lifetime of healthy vision. The three most common ocular diseases we find ourselves addressing are cataracts, glaucoma and macular degeneration. Of these three conditions, the one that can be most visually debilitating and aggressive is age-related macular degeneration (AMD).
Fortunately for optometry, some of our newest retinal technologies have provided us enhanced diagnostic capabilities for detecting AMD earlier, and even more importantly, provided essential information to use as a tool for AMD prevention. I have incorporated two of these technologies into my fullscope, primary eye care practice, and have found them to be invaluable in educating my patients on what this sight threatening condition is and how to reduce their patients risk for long-term vision loss.
The technology we have utilized for educating and identifying risk factors for AMD is the QuantifEYE unit (ZeaVision, LLC). QuantifEYE is a quick, easy test that measures macular pigment optical density (MPOD) by flicker photometry. Studies have shown that a low amount of macular pigment can put patients at a higher risk for vision loss from AMD. Other risk factors include ultraviolet exposure, smoking, obesity and, as previously mentioned, family history, gender and race. Since Caucasian females are at greater risk, we recommend this testing routinely for all Caucasian females 21 and over and, of course, any other patients with two or more risk factors. I say to my patients that they cannot change their gender, ethnicity or family history, but, if they know they have a lower MPOD score, this is a risk factor they can do something about. Diets consisting of foods rich in antioxidants and/or consumption of nutritional supplements have been shown to be beneficial in reducing the severity of AMD.
The QuantifEYE has been available since August 2006, takes up little space and is very technician and patient friendly. From start to finish, the procedure takes approximately three to five minutes. The test need only be performed on one eye; the patient is patched and given a small hand-held device with a button in the center. After being comfortably seated and positioned in the eye-piece, the QuantifEYE unit presents a target, which is a solid blue/gray circle of light, and the patient is asked to click the button when they see the target start to flicker. The target will become solid once again and the patient will continue to click the button each time they see a flicker.
At the end of the testing, the MPOD is measured in a percentage score. Those patients with a score of over 45% are considered to have a high macular pigment level and a lower risk for AMD. Those patients with a score of 25%–45% are considered mid-range, so other risk factors are taken into account to determine level of risk. Those patients below 25% macular pigment are considered at greater risk for AMD, so vitamin supplementation should seriously be considered.
In our office, we have found the QuantifEYE an invaluable tool that not only identifies patients that can benefit from vitamin supplementation but, as importantly, tracks those patients on high dose supplements to see if indeed it is improving their level of macular pigment, thus reducing their risk for AMD.
Because the QuantifEYE unit is identifying MPOD, which is only a risk factor for AMD, there is no medical code available at this time; insurance does not pay for additional testing related to prevention. My office charges a small usage fee per test of $25; this is extremely affordable for most patients and we have over a 95% acceptance rate.
Testing and tracking a patient’s macular pigment is a great tool in AMD prevention, but we have also utilized another technology to better manage those patients with known, visible macular changes. It has been well documented that patients with clinical signs of dry AMD can at any time convert to the more aggressive wet AMD. In fact, 80% of advanced AMD cases are due to choroidal neovascularization (CNV). Rightly so, we monitor these at-risk patients regularly in our offices and give them home Amsler grids for self-monitoring. Development of CNV can happen very quickly with lesions growing 20 microns per day.1 Unfortunately, as clinicians, we are not diagnosing these lesions soon enough. Most practices pick up CNV conversion 5.5 months after progression when the lesions are about 3,300 microns.2 We know with the great breakthrough with the anti-VEGF treatments, the earlier we pick up conversion from dry to wet leads to better therapeutic outcomes. We have found the Foresee Preferential Hyperacuity Perimeter (Foresee PHP, Notal Vision) an invaluable tool in tracking early growth of CNV.
The Foresee PHP became available in late 2006 but is the second generation unit of the original Preview PHP technology introduced in 2004. The Foresee PHP utilizes hyperacuity perimetry, which is based on Vernier acuity. Because Vernier acuity localizes an object relative to other objects in space, it is 10 times more sensitive than resolution or Snellen acuity.3 We know the Amsler grid has basic flaws when it comes to completion, fixation and crowding. Cortical completion is found with the Amsler grid because our brain learns to “fill in” a full line if small gaps of the grid pattern are developing. Fixation is not truly forced on the Amsler grid since the test is subjective and so there is no feedback for the patient if fixation is off. Crowding is found due to inhibition of neighboring peripheral lines that reduces detection of distortions. The Foresee PHP eliminates the inherit flaws of the Amsler grid and utilizes an “automated perimetry” test of the central 14 degrees of fixation testing over 500 points three to five times each.
The testing is very interactive; the patient holds a stylus in hand and touches the screen after the stimulus (a dot deviation signal or hyperacuity pattern) is presented in a mere 160 millisecond flash. When patients are “flashed” the stimulus (a line with a “bump” in part of it), they simply hit the screen where they perceived the “bump” or broken line to be. As in peripheral automated perimetry (like the Humphrey) the Foresee PHP has fixation control and controlled stimuli, which make the results much more accurate and reliable than traditional Amsler grid testing. The instrument is also technician- and patient-friendly with a test time of approximately 20 minutes from start to finish for both eyes. Repeat testing is recommended every three months. Surprisingly, patients do not complain about the frequency of testing since the test is so much more interactive and enjoyable than peripheral perimetry. Just like peripheral automated perimetry, the test results are compared to a normative base and progression from prior tests is readily identified. The results of the test are easy to interpret and clinical recommendations are given along with test results making it very “doctor friendly.” The Foresee PHP is the only FDA-cleared device to monitor conversion of dry AMD patients to wet AMD patients.
The Foresee PHP test helps determine if treatment for wet AMD is needed, so it can be billed with a visual field code. The current visual field codes are 92082, which is for automated screening, and 92083, which is for automated threshold.
Optometrists who see adult patients in a primary-care setting need to educate them on the growing prevelance of AMD and measures they can take for prevention. To implement ZeaVision’s QuantifEYE, unit there is no large capital outlay. The Foresee PHP has a moderate cost that pays for itself within the first year of ownership. Any optometrist who sees adults should strongly consider both technologies.
Dr. Lowe is currently Director/President of Professional Eye Care Center,
Inc., a private, full-scope primary eye care practice in Chicago. She is a
member of the American Optometric Association, the American Public
Health Association and a fellow of the American Academy of Optometry.
1. Vander JF, Morgan CM, Schatz H. Growth rate of subretinal neovascularization in age-related macular
degeneration. Ophthalmology. 1989 Sep;96(9):1422-6.
2. Olsen TW, Feng X, Kasper TJ, et al. Fluorescein angiographic lesion type frequency in neovascular
age-related macular degeneration. Ophthalmology. 2004 Feb;111(2):250-5.
3. Westheimer G. Visual hyperacuity. Prog Sensory Physiol. 1981;1:1-37
Nutrition and the Eye
By Jeffry Gerson, O.D.
Essentially, retinal imaging tries to diagnose pathology. Although this is of extreme importance in order to afford our patients the opportunity to receive the newest and most effective treatments, it may not be the most important step in saving or maintaining vision. Preventing the need for treatment in the first place is arguably just as important.1
One way to potentially prevent several different ocular pathologies from ever occurring or progressing, in particular age related macular degeneration, is alteration of modifiable risk factors in our patients.2 We, as clinicians, can help influence our patients in reducing these risk factors, which are mainly lifestyle choices. Of these modifiable risk factors (besides smoking which is potentially more difficult to influence) nutrition is a familiar area for most optometrists. While we are aware of the importance of nutrition in prevention of AMD, we may not realize its role in other pathologies, including cataracts.3,4 Many large scale studies, such as the AREDS, and smaller scale studies, such as LAST, point to the importance of proper nutrition when considering AMD.5 These studies show that nutrition can be used as prevention or treatment.
To discuss nutrition in the context of using technology, you can reference several parts of this article to see how macular pigment optical density (MPOD) measuring instruments utilizing heterochromic flicker photometry can help detect one potential risk factor for AMD. The macular pigment is composed of carotenoids, which we take in from our diet. A low MPOD has been postulated to predict higher risk of development of AMD.6 Therefore, intake of these elements is important, whether it be through a healthy diet including plenty of fruits and vegetables, or supplementation with products that have meaningful amounts of these and other important elements.We are also finding out that these same elements that seem to influence MPOD and potential development or progression of AMD also have further value in their affects on vision.
Not only can we use supplementation as prevention, but we also can use it in order to improve vision and retinal function, as has been reported in multiple recent studies.7,8 This allows us to talk to patients about potential improvement and not just maintenance and prevention. From a practical perspective, it may be easier to discuss the possibility of improved visual acuity and contrast than just the risk of progression.
Another important piece of the nutritional puzzle appears to be Omega 3 fatty acids. Numerous recent reports discuss the benefits of adequate intake of this essential nutrient.9 The specific type of fat seems to be important, and this is why Omega-3 fatty acids seem to be beneficial, and other fats appear to be detrimental.We have also learned how Omega 3 can be important to other parts of the body.
Regardless of the exact element to be supplemented, it is important to discuss nutrition with our patients. From a practical perspective, it is easy to ask about diet and current supplementation and smoking status. It is also easy to then educate a patient why these things are pertinent to an eye exam and move forward with recommendations. These recommendations may include alterations to diet, use of multivitamin type products, or eye specific products, such as Ocuvite Adult 50+ (Bausch and Lomb ) or ICAPS (Alcon). The more we have these discussions with our patients, the more likely we are to have a more positive impact.
Dr. Gerson practices at WestGlen Eyecare & Omni Eye Center of Kansas City.
1.Richer S. Is there a Prevention and Treatment Strategy for Macular Degeneration? J Am Optom Assoc. 1993 Dec;64(12):838-50.2. Age Related Eye Disease Study Research Group. A randomoized, placeo-controlled, clinical trial of high dose supplementation with vitamins C and E, beta carotene, and zinc for ARMD and vision loss: AREDS no. 8. Arch Ophthalmol. 2001 Oct;119(10):1417-36.
3. Dherani M, Murthy GV, Gupta SK, et al. Blood levels of vitamin C, carotenoids and retinol are inversely associated with cataract in a North Indian population. Invest Ophthalmol Vis Sci 2008;49(8):3328-35.
4. Associations between plasma levels of vitamins and cataract in the Italian-American Clinical Trial of Nutritional Supplements and Age-Related Cataract (CTNS): CTNS Report #2. Ophthalmic Epidemiol. 2005 Apr;12(2):71-80.
5. Richer S, Stiles W, Statkute L, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry. 2004 Apr;75(4):216-30.
6. Loane E, Kelliher C, Beatty S, Nolan JMThe Rationale and Evidence Base for a Protective Role of Macular Pigment in Age-Related Maculopathy. . Br J Ophalmol. 2008 Jl 21. (Epub ahead of print).
7. Parisi V, Tedeschi M, Gallinaro G, Varano M, Saviano S, Piermarocchi S; CARMIS Study Group. Carotenoids and antioxidants in age-related maculopathy italian study: multifocal electroretinogram modifications after 1 year. Ophthalmology. 2008 Feb;115(2):324-333.e2. Epub 2007 Aug 22.
8. Cangemi FE. TOZAL Study: an open case control study of an oral antioxidant and omega-3 supplement for dry AMD. BMC Ophthalmol. 2007 Feb 26;7:3
9. Seddon JM, Rosner B, Sperduto RD, et al. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001 Aug;119(8):1191-9
New Technologies in My Office
By William Jones, O.D., F.A.A.O.
As a practicing optometrist for more than 30 years, today’s new technology allows me to greatly enhance detection and diagnosis of disease states in my patients. This is extremely important in delivering the quality of eye care and systemic body care that I require in my office. I have a private practice and the bulk of my patients have eye diseases. These new technologies offer many distinct advantages including tests that are easy and quick to perform—something that was not possible just 10 years ago.
One such new technology, the wrist sphygmomanometer, offers an efficient and simple way to obtain my patients’ blood pressure and heart rate. It is a little faster than doing arm sphygmomanometer because the patient doesn’t have to manipulate their clothing to expose their arm. I use this new technology on all my patients, and it is surprising to find patients that are unaware that they have elevated blood pressure or are in denial of the condition.
 Another new technology is the
Kinetic Field Test (KFT, Rush
Instruments). It is a continuous
moving bar visual field test that
tests the central 15 degrees with
a bar pattern that moves in random
directions. When the bars
move across an abnormality, the
encountered boundaries of the
anomaly are perceived as a distortion
in the moving bars pattern.
The computer has a touch
screen, so the patient can draw the distortion being perceived. It is a very
sensitive test due to the fact that the continuous moving bars do not allow
for cortical adaptation or completion. The degree of specificity of the test
is low by its nature. The test is very fast, and it can be completed in about
30 seconds for both eyes (the test is done on a monocular basis). A positive
finding alerts the examiner that further testing is required to rule out a
possible disease state in the visual system.
Another new technology is the
Kinetic Field Test (KFT, Rush
Instruments). It is a continuous
moving bar visual field test that
tests the central 15 degrees with
a bar pattern that moves in random
directions. When the bars
move across an abnormality, the
encountered boundaries of the
anomaly are perceived as a distortion
in the moving bars pattern.
The computer has a touch
screen, so the patient can draw the distortion being perceived. It is a very
sensitive test due to the fact that the continuous moving bars do not allow
for cortical adaptation or completion. The degree of specificity of the test
is low by its nature. The test is very fast, and it can be completed in about
30 seconds for both eyes (the test is done on a monocular basis). A positive
finding alerts the examiner that further testing is required to rule out a
possible disease state in the visual system.
This technology is very good at detecting disease states on the retina. In my practice, it has detected diabetic retinopathy, age-related macular degeneration (AMD), macular holes, epiretinal membranes (ERM) and vitreo-retinal traction. It has also detected severe inferior superficial punctate keratitis (SPK), dense cataracts, the edge a posterior capsulotomy, significant floaters, congenital RPE hamartoma and a quadranopic field defect due to past closed head trauma. It is the only instrument I know of that can detect residual migraine visual field defects, which I have seen in five patients. The test is fast and any technician can easily operate it.
The Preferential Hyperacuity Perimeter, commercially known as the Foresee PHP (Notal Vision), is an additional new instrument I purchased. I have used the PHP to detect CNV on suspected patients who currently have intermediate AMD, and this technology has successfully allowed me to detect the conversion of dry AMD to wet AMD. I would recommend it for the screening or early detection of CNV in patients who are in the intermediate dry AMD stage.
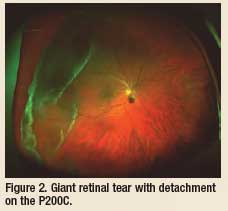 Another new technology I incorporated in my practice is the Panoramic
200C (P200C, Optos), the newest optical device in “ultra-wide field” fundus
imaging. The P200C has a new mirror system that allows for essentially a resolution of the P200C is
20 microns in the 200-
degree Optomap
“Standard Wellness”
image, 14 microns in the
Optomap plus Advanced
Clinical mode, and 11
microns in the ResMax
Advanced Clinical mode.
The resolution is so good
that, in my opinion, no
other imaging instrument
is required in the practice.
It has advanced optical features that allow for eye-steered fundus imagery
that often gets to the pars plana/ora serrata or very close to it. I recently
treated a 19-year-old female patient with suspected pars planitis. With this
new technology, I was able to image the “snow balls” in the vitreous that
were over or just posterior to the inferior ora serrata. In my diabetic
patients, I have been able to detect tiny dot hemorrhages in the posterior
pole, which may have been microaneurysms (no FA was done) and tiny
hemorrhages in the periphery. The P200C is great for seeing large lesions
of the fundus (large tumors, large retinal detachments, numerous lattice
lesions, etc.) that you can only see partially with binocular indirect ophthalmoscopy
or a slit lamp with a precorneal fundus lens.
Another new technology I incorporated in my practice is the Panoramic
200C (P200C, Optos), the newest optical device in “ultra-wide field” fundus
imaging. The P200C has a new mirror system that allows for essentially a resolution of the P200C is
20 microns in the 200-
degree Optomap
“Standard Wellness”
image, 14 microns in the
Optomap plus Advanced
Clinical mode, and 11
microns in the ResMax
Advanced Clinical mode.
The resolution is so good
that, in my opinion, no
other imaging instrument
is required in the practice.
It has advanced optical features that allow for eye-steered fundus imagery
that often gets to the pars plana/ora serrata or very close to it. I recently
treated a 19-year-old female patient with suspected pars planitis. With this
new technology, I was able to image the “snow balls” in the vitreous that
were over or just posterior to the inferior ora serrata. In my diabetic
patients, I have been able to detect tiny dot hemorrhages in the posterior
pole, which may have been microaneurysms (no FA was done) and tiny
hemorrhages in the periphery. The P200C is great for seeing large lesions
of the fundus (large tumors, large retinal detachments, numerous lattice
lesions, etc.) that you can only see partially with binocular indirect ophthalmoscopy
or a slit lamp with a precorneal fundus lens.
The new patient fixation system in the P200C permits a much easier capture of the fundus, especially in “eye steering” mode. Another advance is the small size of the instrument, which is two-thirds the size of the P200. Imagery with the P200C can be performed with or without dilation of the pupil. There is a great advantage to having the patient’s image up on the monitor when you enter the exam room because, if there is an intraocular problem, the exam can be streamlined to the condition at hand.
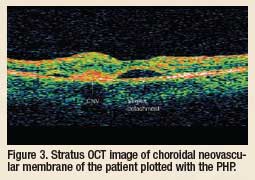 Another new technology in my practice, the Stratus OCT (ocular coherence
tomography, Carl Zeiss Meditec, Inc.), is a scanning light instrument that
produces a crosssectional
view of
intraocular structures.
The scans are
able to determine
retinal thickness
with the cross-sectional
views and
average the cross
sections into thickness
readings. This
instrument is able to
see details of the retina that one cannot see with regular ophthalmoscopy.
It can see neovascularization in AMD, central serous chorioretinopathy,
retinal cystoid spaces, vitreous posterior cortex retinal and disc attachments
that may lead to traction damage and macular holes not visible
with ophthalmoscopy. Optic nerve head and juxtapapillary retinal nerve
fiber layer (RNFL) thickness evaluation for glaucoma and evaluation of
other optic nerve head disease conditions is excellent with this technology.
Determining large physiologic optic disc with large cups and the existence
of glaucoma, due to RNFL loss, is very easy to obtain. I have also used the
RNFL function to determine peripapillary retinal edema in papilledema and
other vascular disease states of the optic nerve head. It is also useful in
detecting the presence of intrapapillary drusen by determining disc elevation
and imaging the drusen themselves.
Another new technology in my practice, the Stratus OCT (ocular coherence
tomography, Carl Zeiss Meditec, Inc.), is a scanning light instrument that
produces a crosssectional
view of
intraocular structures.
The scans are
able to determine
retinal thickness
with the cross-sectional
views and
average the cross
sections into thickness
readings. This
instrument is able to
see details of the retina that one cannot see with regular ophthalmoscopy.
It can see neovascularization in AMD, central serous chorioretinopathy,
retinal cystoid spaces, vitreous posterior cortex retinal and disc attachments
that may lead to traction damage and macular holes not visible
with ophthalmoscopy. Optic nerve head and juxtapapillary retinal nerve
fiber layer (RNFL) thickness evaluation for glaucoma and evaluation of
other optic nerve head disease conditions is excellent with this technology.
Determining large physiologic optic disc with large cups and the existence
of glaucoma, due to RNFL loss, is very easy to obtain. I have also used the
RNFL function to determine peripapillary retinal edema in papilledema and
other vascular disease states of the optic nerve head. It is also useful in
detecting the presence of intrapapillary drusen by determining disc elevation
and imaging the drusen themselves.
The Stratus is a time domain OCT, which takes time to scan through a section of tissue. It requires some skill to obtain good scans, and eye movements can affect the results of the scan. The new Spectral or Fourier domain OCTs take instant captures of a block of tissue, so these require less skill level and training to use. Additionally, eye movements usually have little impact on the results of the scan. Any clinician interested in obtaining fine detail information of the retina and optic nerve head will find OCT technology indispensable.
Dr. Jones is in private practice in Albuquerque, N.M.
Old School Clinical Skills and Cutting-Edge Retinal Technologies Improve Patient Outcomes
By Joseph J. Pizzimenti, O.D., F.A.A.O.
As an attending optometrist in an academic health center, I have had the unique opportunity to integrate several retinal technologies into clinical practice. There are numerous features that make new technology attractive and useful to a practice. They are listed in Table 1.
Table 1. Features/Advantages of New Retinal Technology
|
Several factors must be considered when evaluating new retinal technology. See Table 2.
Table 2. Factors to Consider
|
Here is a brief synopsis of our instrumentation here at The Eye Care Institute.
Scanning Lasers
Retinal thickness measurements and qualitative studies with scanning lasers enable the identification and tracking of structural changes due to various causes, including age-related macular degeneration (AMD), vitreomacular traction syndrome, epiretinal membranes, macular holes, and various “edematous” retinopathies.
For the past seven years, Optical Coherence Tomography (OCT) has been my “go-to” instrument for cross-sectional posterior segment imaging and quantitative analysis. I currently use the Cirrus OCT (Carl Zeiss Meditec, Inc.). The Cirrus uses a spectrometer as a detector in conjunction with a stationary reference mirror. The lack of moving parts facilitates exceptional image acquisition speed.1 (Figure 1)
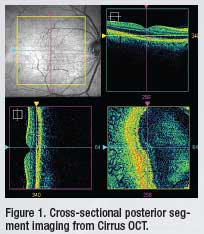 This spectral domain technology
produces high-resolution 2-D and 3-D images that help me to distinguish
between subtle pathological changes and normal anatomic variations.
OCT has enhanced my ability to diagnose and manage myriad
retinal conditions and has revolutionized my evaluation of the vitreoretinal
interface.
This spectral domain technology
produces high-resolution 2-D and 3-D images that help me to distinguish
between subtle pathological changes and normal anatomic variations.
OCT has enhanced my ability to diagnose and manage myriad
retinal conditions and has revolutionized my evaluation of the vitreoretinal
interface.
The HRT-3 Retina Module (Heidelberg Engineering) uses confocal scanning laser ophthalmoscopy technology to evaluate the entire retina. The HRT Edema Index is a relative indicator of fluid accumulation based on changes in light reflectance. Index values over 2.0 are highly suspicious. The HRT-3 uses TruTrack™ technology to check and align the images, remove images with questionable quality, and combine the sets into one 3-D composite, providing Reflectance and Thickness maps.2
The RTA-5 (Talia/Marco) offers the ability to quantitatively document anatomical changes in retinal and subretinal tissues by measuring thickness variations and topographic changes of the chorioretinal interface. Data acquired by the RTA-5 is presented as color-coded 2-D and 3-D thickness and topography maps, deviation probability maps (from a normative database), numerical values, interactive 3D cut sections, and digital fundus images.3
Fundus Photography The Nidek 3Dx Stereo Fundus Camera and 3Dx/F Fluorescent Stereo Fundus Camera are available for stereoscopic macular imaging. The 3Dx has the capability for stereo color photography and fluorescein angiography.4
For general fundus photography, we have a Canon Digital Retinal Camera. The camera provides high-resolution color, red free, and fluorescein angiography imaging. It has user-friendly control software to achieve excellent detail, contrast, color, and archiving.
Functional Macular Testing With Foresee PHP
For the past several years, I have implemented Foresee Preferential Hyperacuity Perimetry (PHP) (Notal Vision/Sightpath) to monitor patients with dry AMD. This instrument is designed to detect early conversion to the wet form of the disease.5 Like glaucoma, AMD is a condition best monitored by both structural and functional testing.
Macular Pigment Optical Density
Thanks to my QuantifEYE system (ZeaVision), I can now measure the amount of macular pigment on my patients. The QuantifEYE uses Heterochromatic Flicker Photometry (HFP) to quantify macular pigment. HFP uses flickering blue and green light targets to yield a measurement reported in density units as Macular Pigment Optical Density (MPOD). Lower MPOD can be associated with increased risk for AMD.
Putting it into Practice
Table 3 lists the appropriate CPT codes for the instruments that I use.
Table 3. Codes
|
“Old School” Skills
With all these great “toys” available to me, it would be easy to get carried away with the hi-tech gear. But of course, excellent patient care is not just about having the latest technology. Here are some thoughts on “lowtech” optometry.
Until the invention of direct ophthalmoscope by von Helmoltz in 1851, the living retina was not visible.6 Simply put, I love the direct ophthalmoscope. It is quick and easy, with good examiner control and 25X magnification. I use it with the red-free filter to detect microaneurysms, small hemorrhages and vitreous opacities.
A skilled clinician is adept at performing and interpreting the results of fundus biomicroscopy. The use of a contact or non-contact fundus lens in conjunction with the slit lamp is a powerful way to evaluate the central, mid-peripheral and peripheral retina, as well as the optic disc.
Of course, no posterior segment examination is complete without binocular indirect ophthalmoscopy through a maximally-dilated pupil. Scleral indentation is a specialized skill that enables viewing of the peripheral retina in profile, yielding useful information about retinal breaks and other clinical entities.
Conclusions
No scanning laser or other futuristic technology can replace the clinical examination and diagnostic skills of an excellent optometrist. An integration of “old school” clinical skills with new retinal technologies may enable the clinician to detect disease earlier, leading to more timely treatment and improved visual outcomes.
It’s not about the technology—it’s about our patients’ visual health and quality of life.
Dr. Pizzimenti is an Associate Professor at Nova Southeastern University College of Optometry, The Eye Care Institute, in Ft. Lauderdale, FL. He is a frequent author and speaker on ocular and oculosystemic disease.
1. Van Velthoven MEJ, Faber DJ, Verbraak FD, et al. Recent developments in optical coherence tomography for imaging the retina. Prog Retin Eye Res 2007 Jan;26(1):57-77.2. Kisilevsky M, Hudson C, Flanagan JG, et al. Agreement of the Heidelberg Retina Tomograph II macula edema module with fundus biomicroscopy in diabetic maculopathy. Arch Ophthalmol 2006 Mar;124(3):337-42.
3. Shahidi M, Blair NP, Mori M, et al. Retinal topography and thickness mapping in atrophic age related macular degeneration. Br J Ophthalmol 2002 Jun;86(6):623-6.
4. Greenfield DS, Zacharia P, Schuman JS. Comparison of Nidek 3Dx and Donaldson simultaneous stereoscopic disk photography. Am J Opthalmol 1993 Dec 15;116(6):741-7.
5. Loewenstein A, Malach R, Goldstein M, et al. Replacing the Amsler grid: a new method for monitoring patients related macular degeneration. Ophthalmology 2003 May;110(5):966-70.
6. Helmholtz H von, 1867/1962 Treatise on Physiological Optics volume 3 (New York:Dover, 1962); English translation by J P C Southall for the Optical Society of America (1925) from the 3rd German edition of Handbuch der physiologischen Optik (Hamburg: Voss, 1910; 1st edition; Leipzig: Voss, 1867). Figure 1. Cross-sectional posterior segment imaging from Cirrus OCT.

