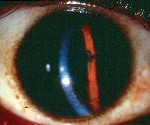
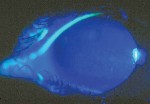
Women undergo a tremendous number of changes, both systemic and ocular, throughout pregnancy. Here, well describe the commonand uncommonchanges that can affect the health and vision of a pregnant patient (and sometimes the fetus, too).
Systemic Changes
Hormonal changes are among the most prominent systemic changes in pregnant women. The placenta, maternal endocrine glands and the fetal adrenal glands combine their productivity to make a high-powered hormone factory.
Metabolic, hematologic, cardiovascular and immunologic changes are also prevelant.1 Pregnancy is potentially diabetogenic.2 Also, the immune state is suppressed, leaving the pregnant woman more susceptible to serious immunological disorders.1
Ocular Changes
Many physiological changes occur in the eye during pregnancy. However, the eye reverts back to pre-pregnancy state within several months after delivery. Intraocular pressure (IOP), the cornea and visual fields are most commonly affected. Changes in accommodation, skin and conjunctival blood vessels have also been noted.
IOP. One benefit of pregnancy is a decrease in IOP, which can occur throughout pregnancy and persists for several months postpartum.3-6 Several causes may explain this phenomenon: increased facility of aqueous outflow, increased uveoscleral outflow and decreased episcleral venous pressure.3,7-9 Pre-existing glaucoma has been found to improve during pregnancy, and initial detection of glaucoma is unlikely during pregnancy.4
Corneal changes. Pregnant patients may experience slightly blurred vision and discomfort with contact lenses that previously fit comfortably.1 A measurable but slight increase in corneal thickness due to edema occurs during pregnancy.10 Corneal sensitivity tends to decrease, with the largest changes late in pregnancy.10,11
Due to variations in thickness, the refractive index of the cornea may be altered. So, postpone changes in prescription and fitting of contact lenses until several weeks postpartum.10
Another corneal change during pregnancy: the appearance of Krukenberg spindles that tend to decrease in size during the third trimester and postpartum. They are not accompanied by other findings of pigment dispersion, such as increased angle pigmentation and iris transillumination defects.11
Visual field loss. Reports differ about the types and mechanisms of visual field loss that might occur during pregnancy. But any symptomatic field loss warrants further investigation.1,12 Even though most of the studies concerning this topic are old, dating back to the 1930s, investigations have shown bitemporal loss, concentric constriction, enlarged blind spots and no detectable changes.12-17 The changes that occurred were asymptomatic and found only due to the investigations. All changes reversed postpartum.1,12
Accommodative changes. Transient accommodative loss has been noted both during and after pregnancy. Accommodative insufficiency and paralysis have been documented in association with lactation.18
External changes. These include a decrease in the number of conjunctival capillaries, along with an increase in the granularity of conjunctival venules.12 Chloasma (or mask of pregnancy), a hormonally mediated process, is characterized by increased pigmentation around the eyes and cheeks.2,12 Spider angiomas, a type of telangectasia, commonly develop during pregnancy on the face and upper body.2 All these external changes resolve postpartum.1,2,12
Keep the following conditions in mind when examining women in their child-bearing years:
Diabetic retinopathy. When examining a pregnant patient (or one planning to become pregnant) who suffers from diabetes, education goes a long way in preventing progression. Proper control of blood glucose is essential to both maternal and fetal well being. Pregnancy influences the natural progression of diabetic retinopathy (DR), so a comprehensive examination with regularly scheduled follow-up is essential.19-25
Diabetic changes that occur during pregnancy are similar to traditional findings (figure 1). There is no difference in the grading procedures. Gestational diabetes (diabetes mellitus that occurs during pregnancy) has not been associated with the development of retinopathy.22 The greatest concern for women with gestational diabetes is that they are at risk of developing type 2 diabetes within five yearsapproximately 35% incidence.
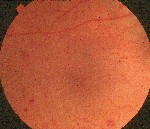 |
| Figure 1. Gestational diabetes has not been linked to diabetic retinopathy. |
Several risk factors for the progression of retinopathy in pregnancy have been documented. Metabolic control, duration of diabetes, baseline severity of retinopathy, retinal blood flow and hyper- tension must be assessed and followed closely during pregnancy.19-23
The treatment of diabetic retinopathy in a pregnant woman is no different than the standard patient, although the timetable for follow-up must be accelerated. Evaluate diabetic patients with minimal or no retinopathy every three months. If baseline retinopathy is moderate, schedule fundoscopic examinations every four to six weeks. If you note progression, two-week evaluations are necessary to determine high-risk characteristics.20
If you note proliferative changes, refer the patient for photocoagulation on a restricted or limited basis. Some studies have found vascular proliferation to be reversible, with regression common postpartum.26 Also, there is a group of unspecified patients in whom retinopathy is particularly aggressive; these patients respond poorly to photocoagulation.27 Unfortunately, there are no factors that may predict this poor outcome. Still, the best course of action is to treat all proliferative changes with photocoagulation prior to pregnancy.19-23
Macular edema with and without proliferative retinopathy may also occur during pregnancy. The edema will regress postpartum in some patients, but it may persist in others, causing long-term visual loss.28
| Case Study: Take Care with Cell and Flare A 32-year-old white female who was six months pregnant presented with complaints of ocular discomfort, tearing, redness and photophobia in her left eye. She had been experiencing these symptoms for the past two days. She is a contact lens wearer and denied extended-wear use. She had removed her contact lenses the previous day but found no relief. She denied experiencing any similar symptoms in the past. The examination revealed cell and flare in the anterior chamber O.S. Other structures of the anterior and posterior segment were not involved.
We diagnosed the patient with unilateral idiopathic nongranulomatous anterior uveitis O.S. Because standard treatment involves the use of at least two class C topical medications (prednisolone acetate 1% and cyclopentolate 1%), we contacted the patients obstetrician for consultation. The obstetrician had no objection. We followed the patient daily, with a goal of eliminating the inflammation and discontinuing medication as soon as possible. We administered one drop of cyclopentolate in the office, and dosed Pred Forte (prednisolone acetate 1%, Allergan) as follows: every hour on day one, every two hours on day two, and q.i.d. on days three and four. Due to the short duration of therapy, minimal tapering was necessary. To reduce systemic absorption, the patient performed digital punctal occlusion for three minutes following installation of medication throughout the treatment process. We kept the obstetrician informed of this patients progress during treatment. This patient did not experience any subsequent ocular symptoms or complications with pregnancy or delivery. |
Pituitary tumor/prolactinoma. Prolactinomas are the most common functioning tumors seen in the pregnant patient.29 These tumors fall into two categories: microadenomas, which are smaller than 10mm, and macroadenomas, which are 10mm or larger.30-33 Hormone fluctuations can stimulate estrogen receptors on the prolactinoma, leading to tumor enlargement.32
Although most pituitary adenomas remain asymptomatic during pregnancy, symptoms have been noted as early as the first trimester. Visual disturbances, headaches and diabetes insipidus are common findings. Patients with macroadenomas tend to have a greater chance of tumor growth and the development of symptoms.34
Pregnant women who have microadenomas should undergo visual field assessment each trimester by confrontation with a red pin and by Goldmann perimetry if they exhibit symptoms. Those who have macroadenomas should undergo visual acuity testing and Goldmann perimetry every six weeks.31 An MRI is warranted if symptoms arise with either tumor.32
Treatment must be instituted immediately when there is evidence of tumor enlargement. Bromocriptine, a dopamine agonist, lowers prolactin, shrinking the tumor.35-37 This medication is considered safe to use during pregnancy and is not associated with increased risk of spontaneous abortion, congenital malformation or long-term postnatal development.38 Glucocorticoids may be used to speed up visual field recovery. If the tumor does not respond to therapeutics, transsphenoidal surgery is advocated during the second trimester, with delivery taking place in the third trimester.32
Sarcoidosis. Sarcoidosis, a systemic granulomatous disease of unknown etiology, frequently affects women during their reproductive years.39,40 Sarcoidosis, including uveitis, can affect women during pregnancy and can occur up to one year postpartum. Pregnancy may have a beneficial effect on the disease, with exacerbation postpartum.41
There are no documented cases of patients whose inactive sarcoidosis was reactivated due to pregnancy. Also, no increased frequency of spontaneous abortions, obstetric complications or congenital abnormalities have been linked to sarcoidosis.39
Several factors have been associated with poor patient prognosis: parenchymal lesions on the chest X-ray, advanced roentgenologic staging, advanced maternal age, low inflammatory activity, need for medications other than corticosteroids and the presence of extrapulmonary sarcoidosis. Even though most pregnant patients who suffer from the disease experience a benign course, they should be evaluated prior to pregnancy for risk and counseled in conjunction with their pulmonary specialist.40
| Case Study: Stork Brings Dry Eye, Too A 27-year-old black female presented with ocular discomfort and blurred vision that began six weeks earlier. She had been wearing the same brand of contact lenses for five years without complaint. She reported no change in wearing schedule or care system during this time. She was a model patient who indicated that she kept to a strict two-week use schedule and wore the lenses 12 to 14 hours a day. Visual examination revealed distance visual acuity of 20/25 O.U. with her current contact lenses. The lenses had adequate movement and centration O.U. Over-refraction produced slight improvement in vision. She removed the lenses, and an evaluation of the cornea revealed subtle superficial punctate keratitis and a decreased tear break-up time in both eyes.
We asked the patient to discontinue contact lens use for several days and begin therapy with artificial tears q.i.d. Upon restarting contact lens use, the same symptoms occurred. The patient returned for follow-up one week later and asked if pregnancy could be the cause of her recent contact lens issues. She revealed that since her last visit, she found out she was pregnant. Armed with this new information, we diagnosed her with pregnancy-induced dry eye syndrome. We fit her with a different contact lens that had a higher water content. The new contact lenses provided better comfort and vision for the patient. We educated the patient that her vision may fluctuate throughout pregnancy, but that she should immediately report any large shifts in vision or loss of vision. She continued to use the new contact lenses throughout her pregnancy and postpartum without incident. |
Graves disease. Graves disease is the most common cause of hyperthyroidism in pregnancy. Ophthalmic signs and symptoms may or may not occur.32 Graves disease tends to remit late in pregnancy and relapse postpartum.42
First, diagnosis during pregnancy can be difficult because many signs and symptoms of hyperthyroidism are common findings during pregnancy, namely shortness of breath, palpitations, weight gain and heat intolerance.43 Preterm delivery, perinatal mortality and maternal heart failure are more common in thyrotoxic women.44,45
Mild cases may be monitored, but moderate to severe cases must be treated. Thyroid inhibitorspropylthiouracil (PTU), methimazole and carbimazoleall cross the placenta and are excreted in breast milk, but the drug of choice is PTU.42
Pathologic Changes
Central serous chorioretinopathy (CSCR). CSCR is a macular disorder characterized by a localized serous retinal detachment. It most commonly affects young middle-aged adults ages 20 to 45. It afflicts men more than women at a 10:1 ratio.46-49 Pregnancy is considered a risk factor for the development of CSCR.47
CSCR can occur at any time during pregnancy, with patient complaints of blurred vision, metamor- phopsia, color changes and darkening of the central visual field.46 All documented cases show resolution of symptoms following delivery, leaving mottling and clumping of the retinal pigment epithelium.1,48 A white/gray subretinal fibrin deposit may be seen under the CSCR, which resolves spontaneously.49 Recurrence (in the same eye) in successive pregnancies has been documented.50
Treatment, as with traditional CSCR, is supportive. Focal laser photocoagulation can be postponed because of the strong likelihood that the condition will resolve following pregnancy.
Pseudotumor cerebri (PTC). PTC is a syndrome of increased intracranial pressure without hydrocephalus. It is seen in women of childbearing age and more commonly in obese women. Frequent symptoms include headaches, vomiting, tinnitus, diplopia and visual disturbances, including transient visual obscurations, visual field loss and loss of central visual acuity.51,52
The etiology of this condition remains unknown, but recent studies disprove earlier beliefs that PTC is found in higher numbers in pregnant women.53-55 Most patients develop symptoms in the first trimester, although symptoms may be seen throughout pregnancy. Some women relapse with subsequent pregnancies.51
Once diagnosed, the decision to treat is based on visual acuity and visual field loss. Optic atrophy and permanent visual field loss can be found following resolution, but pregnancy does affect the eventual visual outcome of patients.1,52,56 Even though patients with PTC do not have a higher rate of spontaneous abortion vs. the general pregnant population, lumbar puncture is considered risky.1,57 Steroids are used by many obstetricians, while dehydrating agents are contraindicated.57,58 Optic nerve sheath decompression and lumboperitoneal shunting are acceptable during pregnancy.51
Hypertensive disorders. Pregnancy-induced hypertension (PIH) can be classified as preeclampsia and eclampsia. Preeclampsia is a triad of hypertension, edema and proteinuria. Eclampsia includes this triad, but also accounts for the incidence of convulsions.1 Another category is pregnancy-aggravated hypertension, which is preeclampsia or eclampsia superimposed upon chronic hypertension.59
Complaints include blurred vision, photopsia, loss of vision (central and total) and diplopia.1,60-62 Ophthalmoscopic findings include reduced arteriole to venule ratio, acute hypertensive retinopathy (figure 2), serous exudative retinal detachment and choroidal infarcts.63 The severity of preeclampsia and fetal mortality have been associated with the degree of retinal arteriolar narrowing.62
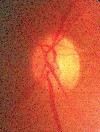 |
| Figure 2. Hypertensive retinopathy. |
Serous exudative detachments have been reported to occur in both preeclampsia and eclampsia at a rate of 1% to 2% and 10%, respectively.64,65 The detachments are usually bilateral and are thought to result from underlying choriocapillaris obstruction.62 Resolution typically occurs within a few weeks postpartum, but long-term visual changes may occur due to retinal pigment epithelium changes or optic atrophy.1,64,66,67
Blindness associated with preeclampsia has been attributed to many causes. Cortical blindness, a temporary or permanent visual impairment caused by the disturbance of the posterior visual pathways and/or the occipital lobes of the brain, has been connected with preeclampsia.68-70 The visual loss resolves postpartum.62 Other causes of blindness associated with preeclampisa include papillophlebitis, retinal artery and vein occlusion, optic neuritis, ischemic optic neuropathy, and thrombosis of the central retinal artery.71-73
Medications
The treatment of both ocular and systemic diseases becomes more complicated in a pregnant patient. You not only have to concern yourself with treating the disease, but also with the effects medications can have on the fetus. In 1979, the Food and Drug Administration established five categories that classify a drugs teratogenic effects on the fetus:74
Category A. Adequate, well-controlled studies demonstrate no risk of teratogenic effects on the developing fetus.
Category B. Animal studies show no risk to the fetus, but there are no adequate, well-controlled studies in pregnant women. Or, some animal studies have shown fetal risk factors, but adequate, well-controlled human studies do not show the same risks.
Category C. Animal studies have found teratogenic or embryocidal effects, but the same findings are not reflected in adequate, well-controlled studies of pregnant women. Or, there are no adequate animal or human studies available.
Category D. These drugs have been proven to have severe fetal risks but are considered for treatment of life-threatening diseases when safer drugs are ineffective.
Category X. These drugs are contraindicated in women who are or may become pregnant. These drugs have greater risks to the fetus, and the teratogenic effects outweigh the benefits.
The diagnostic drugs that most optometrists use regularlyproparacaine, phenylephrine and tropicamideare all category C drugs. So, defer dilation during pregnancy unless a systemic or ocular complication occurs. If required, punctal occlusion and closing the eyes for several minutes following installation of the drops is advised. Avoid phenylephrine due to systemic side effects, and homatropine/atropine due to the risk of birth defects.75 If possible, check IOP via non-contact tonometry to avert the use of proparacaine.
Caring for a pregnant patient can seem a daunting task at first, but it should not be if you are armed with knowledge concerning prevalent diseases and their treatments. While many common systemic and ocular changes have been documented, there is still a risk for many other diseases to occur and/or become exacerbated during pregnancy.
The most important aspect of treatment is to include other medical professionals, including the obstetrician, neurologist, ophthalmologist and endocrinologist. When working as a team, the care of the pregnant patient with ocular complaints becomes manageable.
Drs. Taub and Bartuccio are full-time faculty members in the pediatrics/binocular vision department at Nova Southeastern University College of Optometry, in Fort Lauderdale, Fla. Dr. Davis is a part-time faculty member at Nova Southeastern and a full-time private practitioner in South Florida.
1. Sunness JS. The pregnant womans eye. Surv Ophthalmol 1988 Jan-Feb;32(4):219-38.
2. Pritchard JA, MacDonald PC, Grant NF. Williams Obstetrics. 17th ed. Norwalk, Conn.: Appleton-Century-Crofts, 1985:188-91.
3. Becker B, Friedenwald JS. Clinical aqueous outflow. AMA Arch Ophthalmol 1953 Nov;50(5):557-71.
4. Imre J. Pregnancy and the eye, their endocrinological relations. XV Concilium Ophthalmol Egypt III 1937;213-26.
5. Kass MA, Sears ML. Hormonal regulation of intraocular pressure. Surv Ophthalmol 1977 Nov-Dec;22(3):153-76.
6. Horven I, Gjonnaess H. Corneal indentation pulse and intraocular pressure in pregnancy. Arch Ophthalmol 1974 Feb;91(2):92-8.
7. Paterson GD, Miller SJ. Hormonal influence in simple glaucoma: a preliminary report. Br J Ophthalmol 1963 Mar;47:129-37.
8. Phillips CI, Gore SM. Ocular hypotensive effect of late pregnancy with and without high blood pressure. Br J Ophthalmol 1985 Feb;69(2):117-9.
9. Wilke K. Episcleral venous pressure and pregnancy. Acta Ophthalmol Suppl 1975;(125):40-1.
10. Millodot M. The influence of pregnancy on the sensitivity of the cornea. Br J Ophthal 1977 Oct;61(10):646-9.
11. Riss B, Riss P. Corneal sensitivity in pregnancy. Ophthalmologica 1981;183(2):57-62.
12. Somani S. Pregnancy, special considerations. www.emedicine.com/oph/topic747.htm. (10 Jan 2005)
13. Carvill M. Bitemporal contraction of the fields of vision in pregnancy. Am J Ophthalmol 1923;6:885-91.
14. Finlay CE. Bitemporal contraction of visual fields in pregnancy. Arch Ophthalmol 1923;52:50-5.
15. Finlay CE. Visual field defects in pregnancy. Arch Opthalmol 1934;12:207-19.
16. Johns JP. The influence of pregnancy on the visual field. Am J Ophthalmol 1930;13:956-67.
17. Abramowicz I. On bitemporal contraction of the visual field in pregnancy. Br J Ophthalmol 1927;11:17-27.
18. Duke-Elder S. System of Ophthalmology. Vol. 7. St. Louis: CV Mosby, 1971:703.
19. Oguz H. Diabetic retinopathy in pregnancy: Effects on the natural course. Semin Ophthalmol 1999 Dec;14(4):249-57.
20. Best RM, Chakravarthy U. Diabetic retinopathy in pregnancy. Br J Ophthalmol 1997 Mar;81(3):249-51.
21. Sheth BP. Does pregnancy accelerate the rate of progression of diabetic retinopathy? Curr Diab Rep 2002 Aug;2(4):327-30.
22. Soubrane G, Coscas G. Influence of pregnancy on the evolution of diabetic retinopathy. Int Ophthalmol Clin 1998 Spring;38(2):187-94.
23. Aiello LP, Cahill MT, Wong JS. Systemic considerations in the management of diabetic retinopathy. Am J Ophthalmol 2001 Nov;132(5):760-76.
24. Moloney JB, Drury MI. The effect of pregnancy on the natural course of diabetic retinopathy. Am J Ophthalmol 1982 Jun;93(6):745-56.
25. Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 1984 Apr;102(4):527-32.
26. Serup L. Influence of pregnancy on diabetic retinopathy. Acta Endocrinol Suppl (Copenh) 1986;277:122-4.
27. Conway M, Baldwin J, Kohner EM, et al. Postpartum progression of diabetic retinopathy. Diabetes Care 1991 Nov;14(11):1110-1.
28. Sinclair SH, Nesler C, Foxman B, et al. Macular edema and pregnancy in insulin-dependent diabetes. Am J Ophthalmol 1984 Feb;97(2):154-67.
29. Frohman LA. Pituitary tumors in pregnancy. Endocrinologist 2001 Sep/Oct;11(5):399-406.
30. Babu Segu V. Prolactinoma. www.emedicine.com/med/topic1915.htm. (10 Jan 2005)
31. Randeva HS, Davis M, Prelevic GM. Prolactinoma and pregnancy. BJOG 2000 Sep;107(9):1064-8.
32. Nader S. Thyroid disease and other endocrine disorders in pregnancy. Obstet Gynecol Clin North Am 2004 Jun;31(2):257-85, v-vi.
33. Molitch ME. Pituitary diseases in pregnancy. Semin Perinatol 1998 Dec;22(6):457-70.
34. Molitch ME. Pituitary tumors and pregnancy. Growth Horm IGF Res 2003 Aug;13 Suppl A:S38-44.
35. Bergh T, Nillius SJ, Enoksson P, Wide L. Bromocriptine-induced regression of a suprasellar extending prolactinoma during pregnancy. J Endocrinol Invest 1984 Apr;7(2):133-6.
36. Crosignani P, Ferrari C, Mattei AM. Visual field defects and reduced visual acuity during pregnancy in two patients with prolactinoma: Rapid regression of symptoms under bromocriptine. Case reports. Br J Obstet Gynaecol 1984 Aug;91(8):821-3.
37. Honda Y, Takahashi A. Importance of visual field tests of patients with pituitary adenoma during pregnancy and post-partum lactation. In: Heijl A, Greve EL (eds). Procedures of the 5th International Visual Field Sysmposium 1985. Dordrecht, Netherlands: DW Junk, 1985:227-31.
38. Krupp P, Monka C. Bromocriptine in pregnancy: Safety aspects. Klin Wochenschr 1987 Sep 1;65(17):823-7.
39. Selroos O. Sarcoidosis and pregnancy: A review with results of a retrospective survey. J Intern Med 1990 Apr;227(4):221-4.
40. Haynes de Regt R. Sarcoidosis and pregnancy. Obstet Gynecol 1987 Sep;70(3 Pt 1):369-72.
41. Mayock RL, Sullivan RD, Greening RR, Jones R. Jr. Sarcoidosis and pregnancy. J Am Med Assoc 1957 May 11;164(2):158-63.
42. Becks GP, Burrow GN. Thyroid disease and pregnancy. Med Clin North Am 1991 Jan;75(1):121-50.
43. Sipes SL, Malee MP. Endocrine disorders in pregnancy. Obstet Gynecol Clin North Am 1992 Dec;19(4):655-77.
44. Davis LE, Lucas MJ, Hankins GD, et al. Thyrotoxicosis complicating pregnancy. Am J Obstet Gynecol 1989 Jan;160(1):63-70.
45. Phoojaroenchanachai M, Sriussadaporn S, Peerapatdit T, et al. Effect of maternal hyperthyroidism during late pregnancy on the risk of neonatal low birth weight. Clin Endocrinol (Oxf) 2001 Mar;54(3):365-70.
46. Sunness JS, Haller JA, Fine SL. Central serous chorioretinopathy and pregnancy. Arch Ophthalmol 1993 Mar;111(3):360-4.
47. Haimovici R, Koh S, Gagnon DR, et al. Risk factors for central serous chorioretinopathy: A case-control study. Ophthalmology 2004 Feb;111(2):244-9.
48. Ko W. Central serous chorioretinopathy associated with pregnancy. J Ophthalmic Nurs Technol 1992 Sep-Oct;11(5):203-5.
49. Gass JD. Central serous chorioretinopathy and white subretinal exudation during pregnancy. Arch Ophthalmol 1991 May;109(5):677-81.
50. Chumbley LC, Frank RN. Central serous retinopathy and pregnancy. Am J Ophthalmol 1974 Feb;77(2):158-60.
51. Evans RW, Friedman DI. Expert opinion: The management of pseudotumor cerebri during pregnancy. Headache 2000 Jun;40(6):495-7.
52. Ellent R. Pseudotumor cerebri during pregnancy. Clin Eye Vis Care 1999 Jan;10(4):189-94.
53. Digre KB, Varner MW, Corbett JJ. Pseudotumor cerebri and pregnancy. Neurology 1984 Jun;34(6):721-9.
54. Kassam SH, Hadi HA, Fadel HE, et al. Benign intracranial hypertension in pregnancy: current diagnostic and therapeutic approach. Obstet Gynecol Surv 1983;38(6):314-21.
55. Giuseffi V, Wall M, Siegel PZ, Rojas PB. Symptom and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri): A case-control study. Neurology 1991 Feb;41 (2(Pt 1)):239-44.
56. Boddie HG, Banna M, Bradley WG. Benign intracranial hypertension. A survey of the clinical and radiological features, and long-term prognosis. Brain 1974;97(2):313-26.
57. Swanson MW. Spontaneous regression of pregnancy associated papilledema. Southern Journal of Optometry 1991;9:26-31.
58. Corbett JJ, Thompson HS. The rational management of idiopathic intracranial hypertension. Arch Neurol 1989 Oct;46(10):1049-51.
59. Pritchard JA, MacDonald PC, Grant NF. Williams Obstetrics, ed. 17. Norwalk, Conn: Appleton-Century-Crofts, 1985;525-560.
60. Dinn RB, Harris A, Marcus PS. Ocular changes in pregnancy. Obstet Gynecol Surv 2003 Feb;58(2):137-44.
61. Dieckmann WJ. The Toxemia of Pregnancy, 2nd ed. St. Louis: CV Mosby, 1952;240-249.
62. Jaffe G, Schatz H. Ocular manifestations of preeclampsia. Am J Ophthalmol 1987 Mar 15;103(3 Pt 1):309-15.
63. Capoor S, Goble RR, Wheatley T, Casswell AG. White-centered retinal hemorrhages as an early sign of preeclampsia. Am J Ophthalmol 1995 Jun;119(6):804-6.
64. Fry WE. Extensive bilateral retinal detachment in eclampsia with complete reattachment. Arch Ophthalmol 1929;1:609-14.
65. Hallum AV. Eye changes in hypertensive toxemia of pregnancy. JAMA 1936;106:1649-51.
66. Ballantyne AJ, Michaelson IC. Textbook of the Fundus of the Eye, 2nd ed. Baltimore: Williams and Wilkins, 1970:182-3.
67. Crowther WL, Hamilton JB. Eclampsia with amaurosis due to detachment of the retina. Med J Aust 1932;2:177-8.
68. Nishimura RN, Koller R. Isolated cortical blindness in pregnancy. West J Med 1982 Oct;137(4):335-7.
69. Beeson JH, Duda EE. Computed axial tomography scan demonstration of cerebral edema in eclampsia preceded by blindness. Obstet Gynecol 1982 Oct;60(4):529-32.
70. Grimes DA, Ekbladh LE, McCartney WH. Cortical blindness in preeclampsia. Int J Gynaecol Obstet 1980 May-Jun;17(6):601-3.
71. McLoone EM, Best RM. Pregnancy-related papillophlebitis. Eur J Ophthalmol 2004 Jan-Feb;14(1):65-6.
72. Carpenter F, Kava HL, Plotkin D. The development of total blindness as a complication of pregnancy. Am J Obstet Gynecol 1953;66(3):641-7.
73. Beck RW, Gamel JW, Willcourt RJ, Berman G. Acute ischemic optic neuropathy in severe preeclampsia. Am J Ophthalmol 1980 Sep;90(3):342-6.
74. Schecter S. The Special Patient Part III: How to care for mothers-to-be. Rev Optom 2002 Mar 15;139(3):63-70.
75. 2005 Physicians Desk Reference. 59th ed. Montvale, N.J.: Thomson, 2005:217.