Optometrists play an important role in the surgical treatment of hyperopic and presbyopic patients. We are responsible for selecting surgical candidates, making the appropriate surgical recommendations and helping patients understand the effect of surgery on their vision. This article discusses the surgical procedures for the treatment of hyperopia and presbyopia that are currently available, those that will be available in the future and which patients are ideal candidates for each procedure.
Hyperopic laser vision correction initially received FDA approval in 2000. Laser systems by Wavelight, VISX, Alcon and Bausch and Lomb have been FDA approved to perform standard hyperopic procedures.1-4 (See Laser Systems Approved for Hyperopic Procedures.)
| Laser Systems Approved for Hyperopic Procedures Allegretto Wave (Wavelight, Erlangen, Germany). This laser operates on a fast frequency (200hz), allowing for a shorter treatment time. This is important because as the stroma dries during the treatment, the effect of the treatment can vary.6 Second, patient topography is used to determine the appropriate amount of energy so that the desired periphery treatment can be obtained. Additionally, this laser has an eye movement tracker. Tracking eye movements is especially important because hyperopic patients experience difficulty seeing the fixation target. According to the FDA clinical trials, 72% of patients achieved 20/20 vision at three months.1 STAR S4 (VISX, Santa Clara, Calif.). The STAR S4 offers beam sizes from 0.65mm to 6.5mm, variable repetition rates, 3-D tracking and automatic centration of treatment to the center of the pupil.7 The Star S4 is FDA approved to perform custom hyperopia treatments. According to the FDA clinical trials, at 6 months, 61% of patients experienced 20/20 or better vision.2 Also, 90% of patients said they were satisfied or very satisfied with their night vision. LADARVision (Alcon, Fort Worth, Texas). The LADARVision excimer laser features a radar eye tracker to track eye movements. This can help maintain more precise alignment of the eye. Alcon has completed its clinical trial for custom hyperopia and is in the process of submitting its data to the FDA. Technolas Zyoptix (Bausch & Lomb, Rochester, N.Y.). The Technolas uses flying spot technology to create a smooth corneal surface. The system is also ergonomically designed for LASIK so that patients experience more comfort during the procedure. According to B&L, in the FDA clinical study, 87.3% of subjects achieved 20/20 or better vision. |
Hyperopic LASIK steepens the cornea by removing tissue in the periphery. However, results are less predictable than with myopic treatments because patients with hyperopia can experience more difficulty with fixation. Also, healing variability can occur because more tissue is removed than with myopic treatments. Best vision is achieved later in the postoperative period.5
Patients whose corneas will become steeper than 49.00D postoperatively are not ideal candidates for hyperopic LASIK because corneas will not stay steeper than 49.00D.6 Other patients in whom the procedure is contraindicated: patients who have developing cataracts and those who have unrealistic expectations.
The best candidates for hyperopic treatments are patients with hyperopia that is less than 4.00D, but who are dependent on glasses or contact lenses for distance vision.
Complications that may occur with hyperopic procedures include infection, striae, dry eye and diffuse lamellar keratitis.
Presbyopia
An estimated 90 million baby boomers in America either have presbyopia or will develop the condition in the next 10 years, according to an April 2004 report by Refractec Inc., Irvine, Calif. These patients will eventually require correction, so this leaves many potential candidates for surgical presbyopic correction.
The surgical methods currently available to correct presbyopia include monovision LASIK, conductive keratoplasty and IOL implantation. Also, research on multifocal ablations is under way. This may be a viable option for our patients in the future.
Corneal treatments for presbyopes and select hyperopes include:
Monovision LASIK. Patients who are considering monovision LASIK or PRK should first experience monovision with contact lenses for at least a week to determine whether they can adapt. During this time, they should experience monovision for all of their visual demands, including work and leisure activities. Those who can adapt are typically the best candidates for the procedure.
 |
| Patients considering monovision LASIK should experience monovision with contact lenses for at least one week to see if they can adapt. |
Based on my personal experience, patients who have high demands for their near or distance vision, such as engineers or police officers, should not be considered for monovision LASIK. These patients will not be very tolerant of less-than-perfect results in each eye.
Postoperatively, if patients vision is less than perfect, they may require an enhancement because their eyes are less dependent on each other than before the procedure. However, many patients are successful with monovision LASIK because theyve already experienced monovision with contacts.
Conductive keratoplasty (Refractec, Irvine, Calif.). Conductive keratoplasty (CK) uses radio waves to shrink collagen and temporarily reshape the cornea. The surgeon applies a hand piece with a small tip to the cornea; this hand piece acts as an antenna to conduct radio waves. A speculum is used for the return path. The tip penetrates about 80% of the peripheral cornea, creating a deep and symmetrical leukoma (opaque white spot). By precisely controlling the amount of energy applied, the surgeon can create reproducible treatments that alter the shape and, therefore, the refractive power of the cornea.
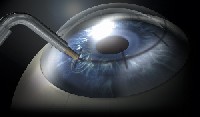 |
| Conductive keratoplasty alters the shape and, therefore, the refractive power of the cornea. |
Conductive keratoplasty received FDA approval in 2002 for the correction of hyperopia in patients past age 40. Last year, the FDA approved NearVision CK for treating presbyopia and hyperopia.8
In presbyopic patients, NearVision CK makes one eye myopic, similar to monovision. However, CK typically results in better outcomes than monovision laser treatments because CK does not make the cornea as steep as monovision LASIK. Therefore, less near-sightedness occurs.
In patients who have undergone CK, their postoperative distance and near uncorrected visual acuity are typically better than what their refraction indicates. Recent data shows that the central 1mm of topography after CK is flatter (i.e., has less plus power) than what most topographers measure. Therefore, we must remember not to be fooled by topography results when caring for patients who have undergone CK.9
One-year data from the FDA trial indicated that 87% of patients who had undergone CK had binocular uncorrected visual acuity of J3 or better.8 These patients did not experience a change in depth perception or contrast sensitivity.
Some regression may occur because the effect of shrinking the collagen diminishes over time.8 So, we must inform patients that future treatments may be necessary.
Ideal candidates for CK are low hyperopes or emmetropes who have less than -1.00D of astigmatism, who are in their late 40s or early 50s and whose vision is not improved by glasses. Patients with very strong near or distance visual demands are not ideal candidates because they may be unsatisfied with less-than-perfect results.
Patients who elect to undergo this procedure may experience some foreign-body sensation the first few days after surgery. Non-steroidal anti-inflammatory drops may improve comfort.
The most significant postoperative complication we should look for here is a decentered treatment. A patient with a decentered treatment will have a decentered apex on corneal topography and experience distorted vision and ghosting of images. Additionally, his vision may not be correctable to 20/20. Custom LASIK may be able to treat this condition.
Multifocal ablations. VISX is currently investigating multifocal excimer ablations as a future treatment of presbyopia. Multifocal ablations will steepen the central cornea and flatten the periphery. Ablation patterns are customized based on patients wavefront readings and pupil size.
In one study of 16 patients who underwent multifocal ablations, results showed a preoperative manifest spherical equivalent (MRSE) of +1.48D with a mean add of +2.25D.10 At six months post-op, 71% of the eyes had 20/20 or better uncorrected visual acuity and 57% of the eyes were J1 or better.
Patients who can adapt to monovision or multifocal soft contact lenses are good candidates for this procedure. Hyperopic patients may also be good candidates for this procedure because the cornea is made steeper for near correction than for distance correction.
Current IOL Options
The current IOL options for presbyopes and select hyperopes include:
Accommodating IOLs. In November 2003, the crystalens (eyeonics, Aliso Viejo, Calif.), became the first accommodative IOL to receive FDA approval.11 The crystalens is a hinged-plate haptic silicone IOL that attaches to the posterior capsular bag. The contraction of the ciliary muscle causes vitreal pressure to move the hinged IOL forward, thereby changing effective lens power. In order for the lens to attach to the posterior capsule, the ciliary muscle must be immobilized with atropine or cyclopentolate for two weeks.
According to the FDA data for the crystalens, 13.6% of patients could see 20/20 at 16 inches, and more than 94% could see 20/20 or better at 24 inches with binocular vision.11
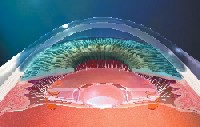 |
| The contraction of the ciliary muscle causes vitreal pressure to move the hinged crystalens IOL (eyeonics inc., Aliso Viejo, Calif.) forward, thereby changing effective lens power. |
Patients who require cataract surgery are typically good candidates for the crystalens. These patients have more realistic expectations than myopic patients, who can see clearly at near without their glasses, sometimes even with cataracts. The crystalens may also be considered for higher hyperopes (more than +4.00D) in their 50s who are considering refractive surgery. LASIK is not recommended for these patients due to the increased risk of reduced best spectacle-corrected visual acuity.6
Patients who are implanted with the crystalens can experience complications that are associated with cataract surgery, such as retinal detachment and endophthalmitis. Additionally, the lens may not adhere to the posterior of the capsular bag. If this occurs, patients will not experience much accommodative effect, and the surgeon may need to reposition the lens.
Multifocal IOLs. The Array Lens (Advanced Medical Optics, Inc., Santa Ana, Calif.) was the first IOL to receive FDA approval for near correction. The Array is a multifocal, foldable silicon IOL that uses five alternating near and far zones to provide increased near vision. According to a meta-analysis of the literature, 41% of patients with the Array can remain spectacle free at all distances.12
The AcrySof ReSTOR IOL (Alcon, Fort Worth, Texas), which received FDA approval in March 2005, uses diffractive optics to allow for distance dominance with a wide pupil and near dominance with a small pupil.13 The optics use the mathematical concept of apodization, which eases the transition between the near and far steps in the lens. According to the FDA data, out of 566 patients with a mean age of 68, 80% of patients implanted with the AcrySof ReSTOR did not require glasses for near or distance vision.13 Additionally, 74% of patients were able to see 20/25 or better after the ReSTOR lens was implanted binocularly.
 |
| The AcrySof ReSTOR IOL (Alcon, Fort Worth, Texas) uses diffractive optics to allow for distance dominance with a wide pupil and near dominance with a small pupil. |
While the Array and ReSTOR are options for patients who require cataract surgery, the lenses can also be implanted in patients who do not have cataracts, namely patients who are not candidates for refractive surgery. These include high hyperopes, up to +12.00D depending on axial length, and patients who have nuclear sclerotic lens changes without reduction in best-corrected visual acuity.
As with multifocal soft contact lenses, patients must accept some compromises in vision, particularly a reduction in contrast sensitivity, when implanted with these IOLs.14 So, when recommending this lens, inform patients that they could experience a loss in quality of vision, especially in low-contrast situations.
Future Lens Options
The future lens options for presbyopes and select hyperopes include:
Dual Optics IOLs. Two dual optic IOLs have been developed
but are not yet FDA approved. The Synchrony (Visiogen, Irvine, Calif.) has a positive-powered anterior optic that is connected with spring-like haptics to a negative-powered posterior optic. The unit sits in the capsular bag. In the unaccommodated state, the tension of the capsular bag positions the two lenses close to one another. With accommodation, the zonules relax, releasing capsular tension and allowing the anterior positive-powered lens to move forward, thus changing the power of the eye. Because the front lens consists of a high power, it needs to move only a small amount to achieve adequate near vision.
 |
| The Synchrony dual-optic IOL (Visiogen, Irvine, Calif.) may be a future lens option for presbyopic and hyperopic patients. |
Some 60 Synchrony IOLs have been implanted in patients outside the United States. Accommodation measured about 2.50D.15 Currently, this is the only result available.
The Sarfarazi Twin-Optic Elliptical Accommodating IOL (Bausch & Lomb, Rochester, N.Y.) is a foldable IOL that employs the same principles as the Synchrony. Clinical studies involving implantation in humans are scheduled to begin within a year.
Other accommodative dual optic IOLs include the FlexOptic IOL (Quest Vision Technologies, Tiburon, Calif.) and the NuLens Accommodating IOL (NuLens Ltd., Herzliya Pitauch, Israel). The FlexOptic IOL undergoes a curvature change that is caused by accommodative effort. Study results for the FlexOptic IOL are not yet available.
The NuLens IOL has a piston-like configuration that compresses a silicone gel-containing central cylinder. When accommodation occurs, the positive power increases by the change in curvature of the anterior surface of the lens. The NuLens IOL has achieved up to 50.00D of accommodation in monkeys.16
Dynamic Optic IOLs. The SmartIOL (Medennium Inc., Irvine, Calif.) is made of a thermodynamic, hydrophobic acrylic material that is similar to that of Medenniums Smartplug punctal plugs. Upon entering the eye, this hard rod becomes a flexible lens shape material. In theory, accommodative forces from the ciliary body will cause anterior and posterior changes in the lens shape, similar to those that occur with the natural lens of the eye. The SmartIOL has not yet been implanted in humans.
Surgical options for hyperopia and presbyopia continue to improve. However, we must seriously consider patient expectations so that we can recommend the appropriate option. We must also make sure that patients clearly understand the benefits and limits of each treatment so that they are satisfied with the procedure.
Dr. Owen is the clinical director for The Laser Center in LaJolla, Calif., and the director of professional development for TLC Laser Eye Centers. Dr. Black is clinical director for The Laser Center in Toronto.
1. Food and Drug Administration. WaveLight Allegretto Wave excimer laser system. Available at www.fda.gov/cdrh/PDF3/
p030008.html. Accessed May 2, 2005.
2. Food and Drug Administration. STAR S4 Excimer laser system with variable spot scanning (VSS) and wavescan wavefront system. Available at www.fda.gov/cdrh/pdf/
p930016s017.html. Accessed May 2, 2005.
3. Food and Drug Administration. Alcon LADARVision excimer laser system. Available at http://www.fda.gov/cdrh/
pdf/p970043s007.html. Accessed May 2, 2005.
4. Food and Drug Administration. Bausch and Lomb Technolas 217A excimer laser system. Available at www.fda.
gov/cdrh/pdf/p990027S004.html. Accessed May 2, 2005.
5. Chayet AS, Magallanes R, Montes M, et al. Laser in situ keratomileusis for simple myopic, mixed and simple hyperopic astigmatism. J Refract Surg 1998 Apr;14(2 Suppl):S175-6.
6. Machat JJ, Slade SG, Probst LE. Predictive formulas for LASIK. In: Machat JJ, Slade SG, Probst LE. The Art of LASIK. 2nd ed. Thorofare, N.J.: Slack Inc., 1999.
7. VISX. CustomVue Technology: STAR S4 laser. Available at www.visx.com/candidates/customvue_technology/s4.laser.
php. Accessed May 4, 2005.
8. Food and Drug Administration. Refractec ViewPoint CK system. Available at www.fda.gov/cdrh/pdf/p010018.html. Accessed May 2, 2005.
9. Holliday J. Optics of near vision conductive keratoplasty: treatment for presbyopia. Data presented at the American Society of Cataract and Refractive Surgery meeting, April 2005; Washington, D.C.
10. Jackson B. Developing the optimal multifocal shape for hyperopic presbyopia. Abstract presented at the European Society of Cataract and Refractive Surgery. Paris, September 21, 2004.
11. Food and Drug Administration. Crystalens model AT-45 accommodating posterior chamber intraocular lens (IOL) P030002. Available at www.fda.gov/cdrh/pdf3/p030002.html. Accessed May 2, 2005.
12. Leyland M, Zinicola E. Multifocal versus intraocular lenses in cataract surgery: a systematic review. Ophthalmology 2003 Sep;110(9):1789-98.
13. Food and Drug Administration. Alcon Acrysof ReSTOR apodized diffractive optic posterior chamber intraocular lenses, Models MA60D3 and SA60D3 - P040020. Available at www.fda.gov/cdrh/pdf4/p040020.html. Accessed May 2, 2005.
14. Steinert RF, Aker BL, Trentacost DJ, et al. A prospective comparative study of the AMO Array zonal-progressive multifocal silicone intraocular lens and a monofocal intraocular lens. Ophthalmology 1999 Jul;106(7):1243-55.
15. Ossna IL. Measurement of accommodation after implantation of dual-optic accommodating IOL. Paper presented at the American Society of Cataract and Refractive Surgery meeting. San Diego, May 4, 2004.
16. Masket S. Accommodating IOLs: emerging concepts and designs. Cataract and Refractive Surgery Today 2004 July;32-6.

