Fuchs’ endothelial dystrophy headlines a group of posterior corneal diseases that cause blurred and hazy vision, glare and halos, progressive corneal edema and, eventually, pain due to corneal bullae. Fuchs’ dystrophy is a bilateral autosomal dominant corneal dystrophy characterized by continuous depletion of endothelial pump cells and abnormal outgrowths on the posterior cornea called guttae (commonly referred to as guttata).1 As endothelial pump cells become fewer and weaker, the cornea swells, leading to a decrease in visual acuity. Corneal transplantation with healthy endothelial cells is the only long-term treatment to restore corneal transparency and reverse corneal edema.2
Recently, lamellar endothelial keratoplasty (EK) has become the standard surgical option for these patients.2,4 According to the Eye Bank Association of America, EK represented more than 40% of corneal grafts in 2010, and this number continues to rise.5 With rapid and predictable visual recovery and lower risk of serious postoperative complications, it’s no wonder surgeons are choosing lamellar EK procedures for endothelial disease in place of penetrating keratoplasty (PKP).6
Identification
Diagnosis of Fuchs’ dystrophy starts with a detailed case history. Depending on disease severity, patients may note blurred or hazy vision, glare, starbursts and difficulty driving at night. A hallmark symptom of Fuchs’ dystrophy is blur upon waking—due to corneal edema—that improves throughout the day.
On slit lamp exam, the endothelium has guttae, the accumulation of which, especially as they become confluent, can render an “orange peel” appearance when the posterior cornea is retroilluminated. Auxiliary testing centers on viewing the corneal edema and imaging the endothelial cells. Corneal edema can be analyzed with both ultrasound pachymetry and tomography. Devices that can perform corneal tomography (not to be confused with optical coherence tomography), such as the Pentacam (Oculus), are helpful for these patients because they use “slit imaging” to analyze both anterior as well as posterior corneal surfaces in near three-dimensions. Contrast this with reflective Placido ring-based topography systems, which precisely measure anterior corneal elevation and surface.7
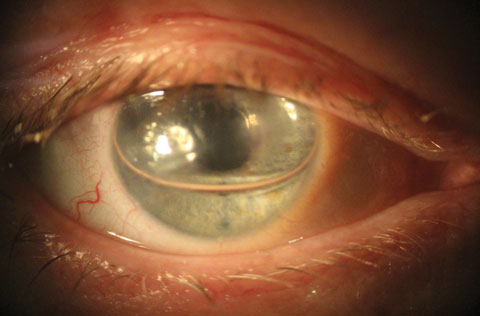 |
| This slit lamp photo shows a patient one day after cataract surgery with DMEK. A gas bubble is covering the pupil with patent inferior PI. |
Specular microscopy is also a big driver in diagnosis and surgical decision making in these patients. The technology provides a quantitative endothelial cell count, as well as qualitative analysis of cell morphology. CellChek (Konan Medical) also measures corneal thickness. A young healthy eye has an endothelial cell density (ECD) of 2,900 cells/mm2 to 3,500 cells/mm2, but as patients age into their 40s and 50s, they experience a natural slow decline in ECD.8 Fuchs’ dystrophy and pseudophakic bullous keratopathy (PBK) accelerate ECD decline. Research shows that ECDs above 500 cells/mm2 are necessary to maintain corneal transparency and ward off cornea edema.8
A critical decision for all eye care providers is when to refer these patients for surgical intervention. Relying only on preoperative refraction, pachymetry, tomography or endothelial cell count can be misleading for a struggling patient. Clinicians must listen to patients regarding their symptoms to develop a better sense of when to refer, as morning blur, or corneal edema, is a big clue for visually significant endothelial disease.
Due to significant advancements in safety and visual outcomes with EK procedures, this option can be confidentially recommended earlier in the disease process.
Two of a Kind
The major endothelial keratoplasty techniques now commonly performed include: Descemet’s stripping endothelial keratoplasty (DSEK/DSAEK) and Descemet’s membrane endothelial keratoplasty (DMEK). The ‘A’ in DSAEK denotes an automated stripping of the donor tissue, which is by far the most common graft preparation method. DSEK involves removing Descemet’s membrane (DM) and endothelium and inserts a graft tissue of posterior stroma, Descemet’s, and endothelium. The corneal graft tissue has extra stroma. In this case (DSEK) stroma sticks to stroma and helps with adhesion. In contrast, DMEK involves removing DM and endothelium and inserts a graft tissue of DM and endothelium. The same layers go in as what the surgeon takes out, so native corneal anatomy is kept the same.
Both procedures start similarly with an inferior Nd:YAG peripheral iridotomy (PI). Because DSEK involves contact between host stromal tissue and posterior stromal tissue from the graft, the sticky stromal tissue in the cornea helps with attachment. In DMEK, the lamellar graft does not have stroma, so host stroma is pressed up against donor DM. The donor graft is inserted into the anterior chamber and slowly unfolded. After the endothelial graft is placed, a gas or air bubble is injected posteriorly to press the tissue into place.
Postoperatively, the patient is instructed to position themselves with their nose pointed to the ceiling to allow gravity to aid in massaging the graft into place. Over the next four to seven days, the bubble will dissipate; surgeon preference dictates the duration of positioning, which often depends on graft appearance.
 |
| This slit lamp photo shows classic endothelial changes in a patient with Fuchs’ dystrophy. Guttata are evident in this photo. |
Management
Following endothelial keratoplasties, management starts immediately after the bubble is placed. Patients should expect hand-motion to count-fingers vision for the first few days as the gas bubble dissipates superiorly above the pupil. This is a critical time to closely monitor the intraocular pressure (IOP), as the bubble can block aqueous movement, leading to an iatrogenic pupillary block angle closure.
Patient education is crucial to ensure they understand the proper positioning with a gas bubble, in addition to inferior PI, and the relief that may come by simply changing from a supine to an upright position. If the PI isn’t functioning or a portion of the bubble moves posteriorly behind the iris, the patient’s IOP can become elevated, which causes an aching periocular pain, nausea and even vomiting. In this case, the patient needs to return to the surgical team so they can, most commonly, create an incision to manipulate the bubble position, known as “burping.”
Since an EK involves a tissue graft, postoperative medication, including a steroid, will help prevent graft rejection episodes. Steroid regimens differ depending on the surgeon, but most commonly patients are started more aggressively on a steroid and tapered over a year to indefinite minimum dosing. Most patients with DMEK can stop steroid medication one year postoperatively, while the treatment paradigm for DSEK is still evolving. In our practice, we stop steroids at one year post-op in both DMEK and DSEK patients, but any patient who develops a graft rejection episode will be restarted on steroid and moved to a tapering schedule with steroids once a day indefinitely.
The postop day one check revolves around IOP and graft position; most importantly, the graft should be anterior to the bubble and starting to adhere. Over the next seven to 10 days, the graft will adhere, and comanaging optometrists should be on the lookout for early graft scrolling or detachments. If the graft is detaching, the surgical team will need to decide on treatment options, such as: monitoring, adding a second air bubble at the slit lamp or returning to the operating room for graft repositioning. Once the graft is attached and functioning properly, the patient is ready for new glasses, which commonly occurs around three months post-op.
DMEK and DSEK have been game-changers for patients with endothelial disease and resulting corneal edema. With DSEK, the cornea is routinely 600µm or greater due to extra stromal tissue inserted on the graft, while DMEK commonly maintains pachymetry postoperatively at around 540µm to 550µm. The varying amount of graft tissue inserted back into the eye differentiates these procedures, and both DMEK and DSEK have advantages worth reviewing.
Comparing DSEK and DMEK
First, and most importantly, patients care about visual acuity. With DSEK, patients are left with their own corneal stroma and posterior stromal tissue on the donor graft. Research suggests this causes abnormal posterior astigmatism, a hyperopic shift and increased higher order aberrations.3 Recently, researchers published comparative results of 100 DSAEK eyes vs. 100 DMEK eyes. The DSAEK group had a preoperative best-corrected visual acuity (BCVA) of 0.41 logMAR (20/50- Snellen) compared with a BCVA of 0.27 logMAR (roughly 20/40+ Snellen) in the DMEK group.9 At the six-month postoperative visit, the DSAEK group’s mean BCVA improved to 0.20 logMAR (20/32- Snellen) compared with a mean BCVA in the DMEK group of 0.11 (20/25- Snellen).9 In a similar study directly comparing DMEK and DSEK, researchers found both groups had a BCVA of approximately 20/100 at three and six months postop.3 In the DSEK group, BCVA improved to 20/60 and 20/40 at three and six months, respectively; in the DMEK group, BCVA was 20/32 at three months and improved to 20/25 at six months.3 These studies suggest that with DMEK, patients recover best visual acuity sooner, and best potential acuity is greater.
Table 1. Endothelial Cell Density by Age8 | |
| Age | Average Endothelial Cell Density (cells/mm2) |
| 10-19 | 2,900-3,500 |
| 20-29 | 2,600-3,400 |
| 30-39 | 2,400-3,200 |
| 40-49 | 2,300-3,100 |
| 50-59 | 2,100-2,900 |
| 60-69 | 2,000-2,800 |
| 70-79 | 1,800-2,600 |
| 80-89 | 1,500-2,300 |
Success rates. Long-term graft success is directly correlated to minimizing endothelial cell loss during tissue implantation and reducing the risk of transplant rejection. Decreased ECD is the principal reason for shortened graft longevity. When researchers compared ECD at the six month follow up between DMEK and DSEK, the results were almost identical: 1,520 cells/mm2 and 1,532 cells/mm2, respectively.3 Other researchers also found no statistical difference in ECD between DMEK and DSEK at six months, and ECD percent loss matched comparative published data.9,10 Recently, researchers analyzed rejection episodes at one year post-op and the prospective risk of rejection at two years in DMEK vs. DSEK. At one year, there was a 0.7% rejection rate in the DMEK group compared with 9% in the DSEK group; DSEK had a two-year prospective risk of 12% compared with 1% in the DMEK group.6 This data parallels other research, which found no rejection episodes in over 100 DMEK eyes at the nine-month follow up.11
Decreasing detachment rates. Graft adhesion is an evolving process for DMEK and DSEK, but detachment rates for both procedures have decreased as the techniques are perfected. In DSEK, the sticky stromal tissue in the cornea helps with attachment, while in DMEK, host stroma is pressed up against donor DM. Although researchers found a much higher partial detachment rate in DMEK at 82% compared with DSEK’s 20%, the study grossly overestimates detachment rates in both surgeries, as the goal was to catch graft dehiscence at the absolute earliest point.3 Another key to interpreting this research: they used air bubble injections to tamponade the new graft into place vs. a gas bubble with sulfur hexafluoride (SF6). SF6 gas has a higher surface tension and a longer half-life inside the eye compared with air and serves a nice compliment to both DMEK and DSEK.4 Investigators measured DSEK outcomes with air bubbles vs. DSEK with SF6 gas bubbles and found that, on graft adhesion, the air group had a 27.2% detachment rate vs. zero detachments in the gas group.4 Interestingly, the air group had a statistically significant higher ECD loss than the gas group.4
As surgeons conquer the learning curve with lamellar transplants, graft detachment rates continue to fall, in part due to the switch to gas bubbles. While DMEK lamellar transplants pose greater risk for graft detachment, many surgeons are reporting rates below 10%.9,10
A Patient-dependent Decision
The added adhesiveness of DSEK benefits patients with a previous glaucoma shunt procedure, as they already have a crowded anterior chamber. The same is true for patients with sutured intraocular lenses (IOLs), anterior chamber IOLs or patients who have undergone vitrectomy. Lamellar transplants, usually DSEK grafts, can safely be used under a previous PKP as well. Finally, in a patient with greatly reduced visual potential, lessening their risk of multiple air/gas additions due to graft detachments may favor DSEK.
A growing population of patients with endothelial diseases, mainly Fuchs’ dystrophy, suffers from reduced vision and corneal edema secondary to unhealthy pump cells. Fortunately, innovation in lamellar transplants offers these patients a long-term and reliable surgical treatment option. As ODs on the front lines, we must educate patients on corneal transplant advancements and maintain our role as pivotal comanagement teammates.
With positive research results and standardized techniques, the excitement grows as surgeons slowly transfer to DMEK as their go-to procedure. It replaces diseased endothelial cells with a near-perfect anatomic replacement to restore corneal transparency and pump functions. When discussing surgical options with patients, you can confidently state that DMEK offers a lamellar transplant with less risk of graft rejection and a faster visual recovery with great endpoint visual acuity.
Dr. Ibach specializes in advanced anterior segment surgery care and pathology at Vance Thompson Vision in Sioux Falls, SD. He is a fellow of the American Academy of Optometry and a member of the American Optometric Association.
|
1. Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuch’s endothelial dystrophy of the cornea. Surv Ophthalmol. 1993;38(2):149-52. 2. Wacker K, McLaren JW, Amin SR, et al. Corneal high-order aberrations and backscatter in fuchs’ endothelial corneal dystrophy. Ophthalmology. 2015;122(8):1645-50. 3. Tourtas T, Laaser K, Bachmann BO, et al. Descemet membrane endothelial keratoplasty versus Descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2012;153(6):1082-90. 4. Acar BT, Muftuoglu O, Acar S. Comparison of sulfur hexafluoride and air for donor attachment in Descemet stripping endothelial keratoplasty in patients with pseudophakic bullous keratopathy. Cornea. 2014;33(3):219-22. 5. Anshu A, Price MO, Tan DT, Price FW. Endothelial keratoplasty: A revolution in evolution. Surv Ophthalmol. 2012;57(3):236-52. 6. Anshu A, Price M, Price F. Risk of corneal transplant rejection significantly reduced with Descmet’s membrane endothelial keratoplasty. Ophthalmology. 2012;119(3):536-9. 7. Ambrosio R, Belin MW. Imaging of the cornea: topography vs tomography. J Refract Surg. 2010;26(11):847-9. 8. Thomas C. Use specular microscopy to diagnose corneal disease. Rev Optom. 2009;146(6):69-74. 9. Hamzaoglu EC, Straiko MD, Mayko ZM, et al. The first 100 eyes of standardized descemet stripping automated endothelial keratoplasty versus standardized descemet membrane endothelial keratoplasty at one institution. Ophthalmology. 2015 Nov;122(11):2193-9.. 10. Terry MA, Straiko, MD, Veldman PB, et al. Standardized DMEK technique: reducing complications using prestripped tissue, novel glass injector, and sulfur hexafluoride (SF6) gas. Cornea. 2015 Aug;34(8);845-52. 11. Cursiefen C, Heindl L, Bachmann B, et al. Immune rejection after isolated transplantation of descemet’s membrane and endothelium (DMEK). Invest Ophthalmol Vis Sci. 2011;52:1155. |

