Glaucoma management requires optometrists stay up-to-date on the latest diagnostic tools, protocols and treatment regimens. New medications, technologies and surgical interventions are quickly becoming integral to the care paradigm.
While clinicians cannot forget the evidence-based findings that have shaped how we diagnose, treat and follow glaucoma patients, many may question the relevance of the landmark studies with the advent of new treatments and technologies. Would the studies have different treatment recommendations if topical rho-kinase inhibitors and nitric oxide (NO)-donating molecules had been available? Would optical coherence tomography (OCT) have changed the outcomes? And, with the boom in minimally invasive glaucoma surgeries (MIGS), is information about filtration surgery even significant? This article revisits several landmark studies and incorporates today’s advances to answer these kinds of questions.
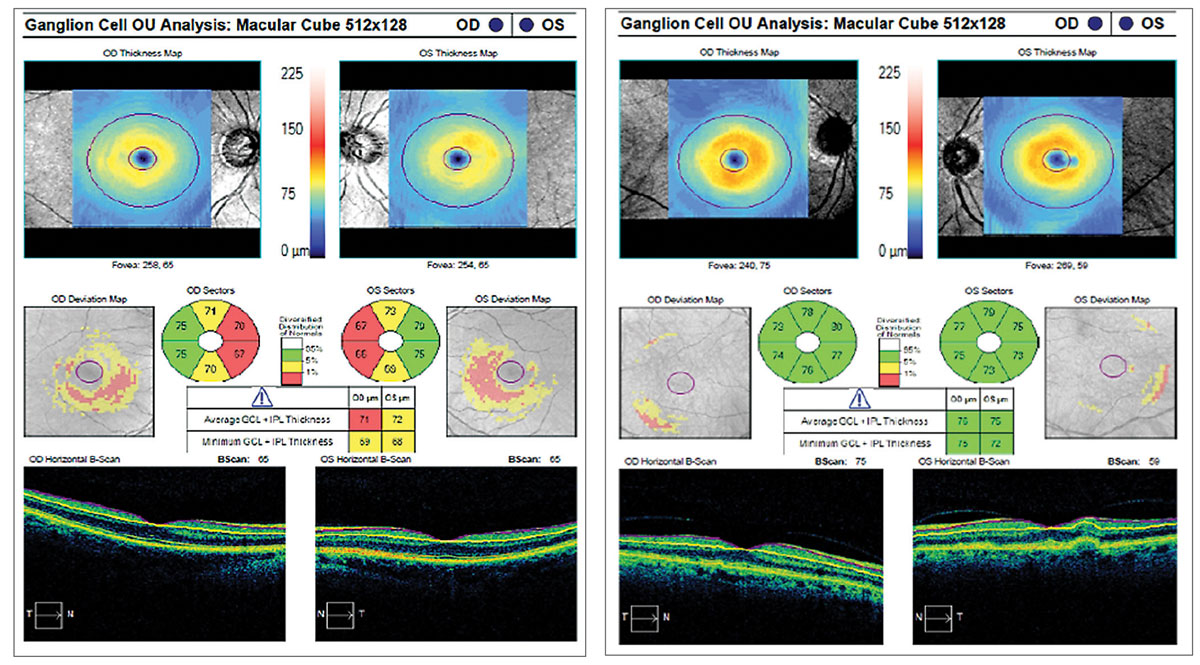 |
The ganglion cell analysis on the left shows a thinned ganglion cell layer secondary to glaucoma, while the right shows a normal ganglion cell layer. |
The Ocular Hypertension Treatment Study (OHTS)
This study from 2002 is one of the first to highlight central corneal thickness (CCT) as a significant structural risk factor for glaucoma.1 The study evaluated 1,636 participants with ocular hypertension, defined as an intraocular pressure (IOP) of 24mm Hg to 32mm Hg in one eye and 21mm Hg to 34mm Hg in the other and no visual field (VF) loss or glaucomatous optic nerve damage.2 The study’s main objectives were to determine the effectiveness of topical treatment in preventing the onset of primary open-angle glaucoma (POAG) in patients with ocular hypertension and to establish baseline demographic and clinical risk factors for the development of POAG in patients with ocular hypertension.2
After five years, researchers found that the incidence of POAG development was 4.4% in the treated group and 9.5% in the untreated group.2 IOP was reduced by 22.5% in the treated group and only 4% in the untreated group, they note.2 The baseline factors associated with a higher risk of developing POAG include older age, elevated IOP, a larger vertical cup-to-disc (C/D) ratio and a thinner CCT.1 Being of African descent was associated with a 59% increased risk of developing POAG; however, the study did not find this to be statistically significant.1 The researchers did note that, at baseline, black participants had a larger mean vertical C/D ratio and a thinner mean CCT compared with other participants.1
The study concludes that the risk of POAG development in ocular hypertensive patients is reduced by almost 50% at five years when treatment is initiated, and some individuals have a higher risk than others.2 Risk assessment is critical in deciding which patients need to be offered treatment.
In a 13-year follow up to OHTS, medication was provided to the original observation group, and their incidence of POAG reached that of the original medication group—suggesting there may not be a significant detriment in delaying treatment in those at lower risk.3
The Early Manifest Glaucoma Trial (EMGT)
This trial, also from 2002, evaluated 255 participants with early-stage glaucoma in at least one eye and a median IOP of 20mm Hg.4 The study objective was to observe the progression of glaucoma after early treatment and after delayed treatment (once progression was observed).4
The group of participants treated with a topical beta-blocker and 360° argon laser trabeculoplasty (ALT) experienced an average IOP reduction of 5.1mm Hg, approximately 25% from baseline, while the control group that received no treatment experienced minimal to no changes in IOP from baseline.4 The researchers found that glaucoma progressed slower in the treated group, and when progression did occur, it occurred 18 months later than it did in the control group.4 The trial concludes that a higher baseline IOP, pseudoexfoliation, bilateral disease, older age, lower ocular perfusion pressure and cardiovascular disease are all risk factors for progression and that with every 1mm Hg reduction in IOP, the risk of progression was minimized by 10%.5
A more recent trial, the Canadian Glaucoma Study, explored this association again and found that every 1mm Hg reduction in IOP reduces the risk of progression by 19%.6 Whether the relationship between IOP and disease progression is completely linear is a topic of debate, but what is clear is that reducing IOP, even by 1mm Hg, is critical to limit progression.
A Surgeon’s PerspectiveConstance O. Okeke, MD, MSCE, a glaucoma specialist and cataract surgeon at Virginia Eye Consultants and assistant professor at Eastern Virginia Medical School in Norfolk, shared her thoughts about the two most recent novel classes of topical medications, NO-releasing agents and ROCK inhibitors. As the trabecular meshwork (TM) ages in glaucoma, resistance to outflow increases, she says. This is the most common pathway manipulated or bypassed in surgical interventions. If these new medications, in their mechanisms of cytoskeletal relaxation of the TM fibers, are started earlier in the disease progression, they may prevent further structural disease of the TM. She adds that they may actually have a positive impact on how certain surgical treatments are used, such as MIGS, since many of those procedures are directed at the TM tissue and work better in patients who have good TM outflow systems that are still intact. “We have evidence that every point matters, that starting prevention early matters and that lowering pressures can relatively halt disease progression,” she says. “But we also know that glaucoma will progress despite a low pressure. I am really excited to see how our newer drugs, the ROCK inhibitors and NO-releasing agents, not only affect the pressure but also affect change in the structure in the outflow pathways. We may find that their impact is much more than IOP reduction, but may have a lasting effect that impacts how we are able to treat glaucoma better with the additional technologies and modalities that we have to offer.” |
The Collaborative Initial Glaucoma Treatment Study
This study, which began enrolling patients in 1999, evaluated 607 participants with newly diagnosed open-angle glaucoma (OAG) and an IOP of at least 20mm Hg to determine whether patients benefit more from initial treatment with topical therapy or trabeculectomy.7
The topically treated group experienced a post-treatment average IOP of 17mm Hg to 18mm Hg compared with 14mm Hg to 15mm Hg in the surgical group.7 At the five-year mark, however, both groups had similar low rates of VF progression.8 The researchers found that patients who have advanced VF loss at baseline, are of older age, are of African descent or have diabetes are more likely to experience VF progression.8
After eight years of follow-up, the study concludes that patients with more advanced VF loss at baseline experience less VF deterioration if they undergo initial treatment with trabeculectomy, supporting the need for early surgical intervention in these patients.9
The Collaborative Normal-Tension Glaucoma Study (CNTGS)
In 1998, researchers evaluated 260 participants with normal-tension glaucoma (NTG), defined as having an IOP of 20mm Hg or less after washout and a VF defect that has not advanced to the point where progression is easily detectable.10 The researchers sought to determine if IOP plays a role in NTG progression and to evaluate the natural tendency of NTG as a disease process.10 Treatment included a combination of topical pilocarpine, systemic carbonic anhydrase inhibitors, ALT and filtering surgery and aimed to reduce IOP by at least 30% from baseline.10
At the five-year mark, 35% of untreated eyes had experienced VF loss progression compared with only 12% of eyes that received treatment.10 Of the untreated eyes, one third had progressed within three years and half within five to seven years.10 The researchers found that those at risk of aggressive progression include women, migraine-sufferers and patients with disc hemorrhages.11
The study concludes that while lowering IOP by 30% slows progression, some NTG cases progress quicker than others, highlighting the importance of identifying patients with aggressive disease and initiating treatment to lower IOP and slow the rate of progression.12
The Advanced Glaucoma Intervention Study (AGIS)
This study, also from the ’90s, evaluated 591 participants with advanced OAG who were undergoing maximum medical therapy, had VF loss and were failing to achieve adequate IOP levels.13
The study aimed to examine the long-term clinical course and prognosis of advanced OAG and compare the outcomes of two sequences of surgical treatments.13 The first treatment arm included a trabeculectomy, followed by an ALT and another trabeculectomy, if necessary (TAT).13 The second treatment arm began with an ALT, followed by a trabeculectomy and a second, if necessary (ATT).13
After seven years, the researchers found that IOP was lower in eyes assigned to the TAT sequence, and those assigned to the ATT sequence suffered from a higher rate of initial therapy failure.13 While African American patients experienced better preservation of visual field and visual acuity with the ATT sequence, Caucasian patients had better preservation with the TAT sequence, suggesting a patient’s race should be taken into account when designing a surgical treatment plan involving trabeculectomy.14
They add that eyes with an initial IOP greater than 17.5mm Hg had statistically significant VF loss compared with eyes that had an IOP less than 14mm Hg, regardless of which surgical treatment course they followed.15 The study concludes that lowering IOP plays a critical role in reducing the progression of VF deterioration in patients with advanced OAG.13-15
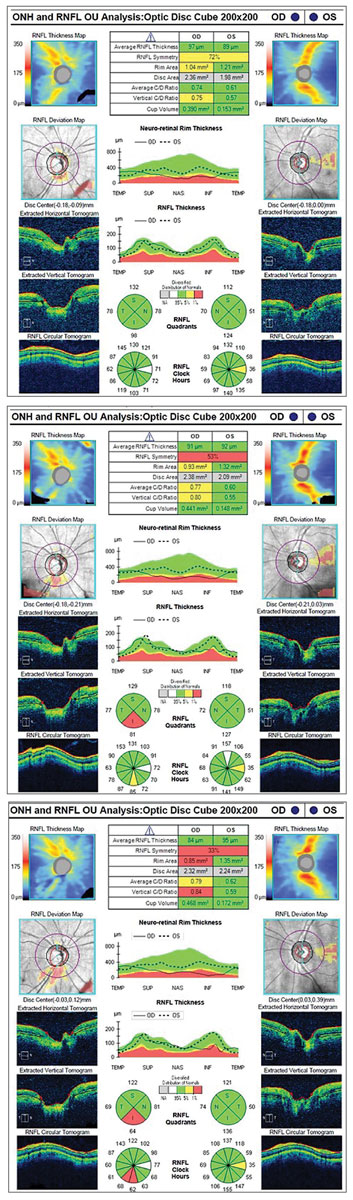 |
| Three years of RNFL OCT scans of a patient with unilateral glaucoma show thinning progression in the inferior quadrant of the right eye. |
That Was Then, This Is Now
While much has changed since these studies shaped our treatment regimens, many of them are still incredibly important for our clinical decision-making process today.
They tell us that only a portion of patients with ocular hypertension go on to develop OAG without any treatment, and treatment reduces this rate significantly. They help us understand which patients are at a higher risk of progression based on clinical risk factors and that early initial treatment to lower IOP is beneficial in preventing or delaying progression.
What If?
These studies, however, come with some pitfalls, especially in light of the tests and treatments available today. The OHTS defined glaucoma progression, similarly to most of the other studies, as VF loss progression measured by Humphrey visual fields or optic disc deterioration noted on photographs. But, if the researchers had included retinal nerve fiber layer (RNFL) loss progression by OCT or OCT ganglion cell analysis as part of their definition of glaucoma progression, the criteria might be different. They may have found more than 9.5% of ocular hypertensive patients experienced progression.
VF testing is the only way to quantify functional loss from glaucoma, but research now shows that up to 50% of the RNFL may deteriorate before a defect is apparent on the visual field.16 RNFL scanning by OCT measures the thickness of the ganglion cell axons around the optic nerve and is now a well-established measurement for detecting early-stage glaucoma and monitoring progression in early to moderate stages.17,18 The macula contains the majority of retinal ganglion cells and, therefore, is primarily where damage takes place during the earliest stage of glaucoma.
OCT analysis has advanced to quantify this layer; ganglion cell analysis can detect glaucomatous damage before a VF defect is observed and can even be beneficial as a parameter for advanced disease detection when RNFL analysis is no longer helpful.19,20
Today, clinicians must fit serial OCT readings into the clinical definition of progression and determine how to administer appropriate treatment when progression is noted on OCT.
The EMGT found that every 1mm Hg matters and that early intervention does slow progression, both of which are still relevant. The standard treatment in the trial, however, was 360° ALT and a topical beta-blocker, which is far from the standard of care today. Although selective laser trabeculoplasty (SLT) and ALT are comparable in their abilities to lower IOP initially, once 360° ALT has been performed, no research shows repeated ALT is as efficacious the second time, while repeated 360° SLT has proven effective.21-23 Thus, the study may have been more successful and achieved a lower percentage of progression had 360° SLT been performed initially and repeated over follow-ups as needed to maintain target IOP instead of one-time 360° ALT and a topical beta-blocker.
Newer topical medications are also a game-changer and are now being used as first-line therapies ahead of the medications used in these studies. Research shows prostaglandin analogs administered once daily are comparable with and even superior in efficacy to timolol taken twice daily.24
Vyzulta (latanoprostene bunod ophthalmic solution 0.024%, Bausch + Lomb) is a combination of prostaglandins and NO-donating molecules that increases aqueous outflow via both the uveoscleral and trabecular meshwork pathways. It can lower IOP by about 1.2mm Hg more than latanoprost and has shown superiority to timolol after three months.25,26 A 1.2mm Hg reduction may seem like a small advantage, but for most patients every 1mm Hg counts.
Rhopressa (netarsudil ophthalmic solution 0.02%, Aerie Pharmaceuticals) is a rho-kinase inhibitor and a norepinephrine transporter inhibitor that increases aqueous outflow through the trabecular meshwork and decreases aqueous fluid production in the ciliary body. While studies have not shown Rhopressa to be superior to latanoprost, it is non-inferior to timolol at baseline IOPs of 21mm Hg to 24mm Hg at three and six months.27,28 This may be a prospective adjunct therapy when secondary agents are needed with a different mechanism of action. This can be especially important for patients with aggressive NTG whose IOP cannot be lowered enough to be beneficial.
Roclatan (netarsudil/latanoprost ophthalmic solution 0.02/0.005%, Aerie Pharmaceuticals) is a combination drop that is pending FDA approval. Researchers suggest that the drop reduces IOP by an average of 1mm Hg to 3mm Hg more than each of its components.29
All of these new treatment options are equal or superior in efficacy to those used in the landmark studies and are becoming more widespread in their use for all stages of glaucoma.
The CIGTS and AGIS found that while filtration surgery can be successful in some patients, associated risk factors exist that clinicians must consider. Today, we have a growing number of MIGS that can be performed before external incision surgery is required. Some MIGS target the conventional outflow pathway, suprachoroidal space and subconjunctival space, providing multiple surgical options for early- to moderate-stage glaucoma patients. While a place still exists for trabeculectomy and tube shunts in surgical glaucoma management, many patients classified as early to moderate stage undergo MIGS and see a reduction in IOP.
These procedures provide a new avenue of surgical intervention with fewer risk factors. As MIGS surgeons become more comfortable with these procedures and their expected outcomes, their use will likely expand into more advanced forms of glaucoma.
Given the significant advancements in topical, laser and surgical options, it is worth questioning whether updating the AGIS would provide more insight into which patients can benefit from initial and repeatable SLT and which may need surgery earlier in the process. For the latter group of patients, MIGS may be an option.
Updating the CIGTS to compare topical treatments with various MIGS options would help clinicians understand when and how to offer these procedures to prevent further glaucomatous damage. Incorporating MIGS into this algorithm, as it creates its own tier in the glaucoma treatment hierarchy, would prove beneficial in providing patients with appropriate options depending on their stage of glaucoma.
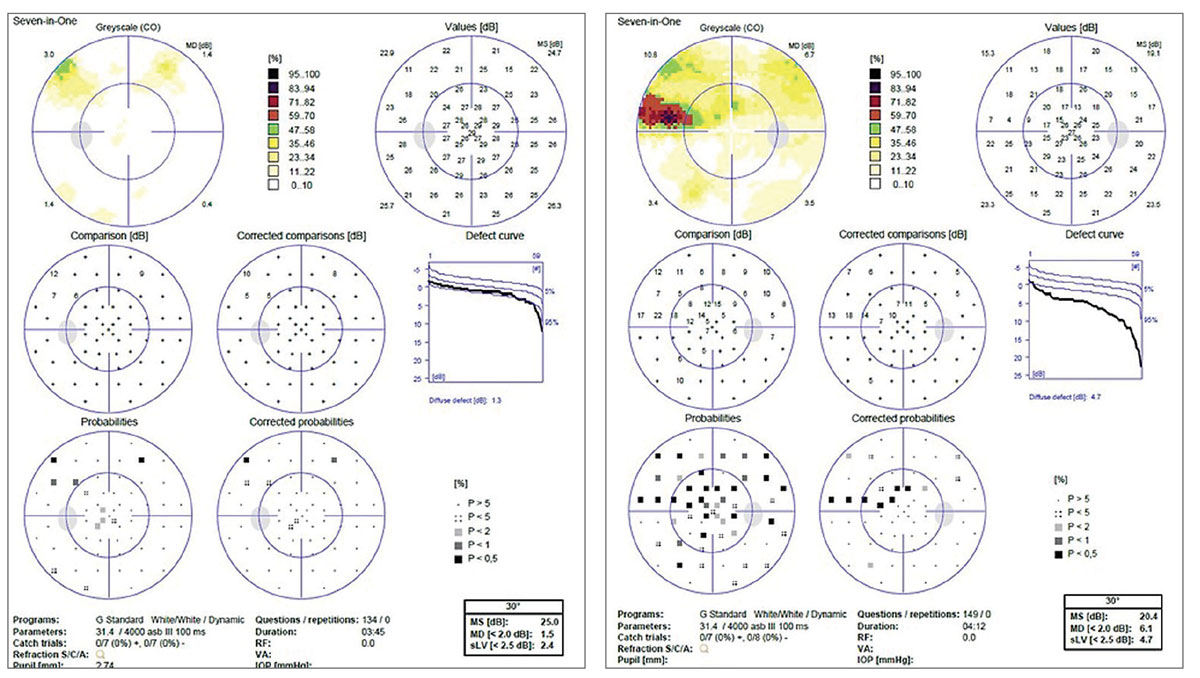 |
This is the visual field of the patient in the RNFL OCTs. The defect correlates with the structural damage seen on OCT scans of the right eye. However, this defect was not present in previous years and was only detected during the most recent RNFL scan. This example showcases the modern definition of progression with OCT. |
Though much has changed in the diagnosis and management of glaucoma in the last two to three decades, landmark studies still provide important evidence to support and guide clinicians. These studies all illustrate the importance of aggressive IOP-lowering to prevent progressive damage to the optic nerve and have driven innovation within the field of glaucoma to what we see today.
Early diagnosis is key to successful long-term management, and diligent IOP-lowering is critical. As technological, pharmacological and surgical advancements continue, we are obligated to stay informed on the newest studies and treatments and combine them with the knowledge we’ve gleaned from these foundational studies to provide the best care for our patients.
Dr. Janes practices at Atlee Gleaton Eye Care in Augusta, ME. She completed her residency training in ocular disease and ocular and refractive surgery at Virginia Eye Consultants.
Dr. Kruthoff is a staff optometrist at Virginia Eye Consultants in Norfolk, VA.
1. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714-20. 2. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines rhat ropical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701-13. 3. Kass MA, Gordon MO, Gao F, et al. Delaying treatment of ocular hypertension. Arch Ophthalmol. 2010;128(3):276-87. 4. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120(10):1268-79. 5. Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1):48-56. 6. Chauhan BC, Mikelberg FS, Balaszi AG, et al. Canadian Glaucoma Study: 2. Risk factors for the progression of open-angle glaucoma. Arch Ophthalmol. 2008;126(8):1030-6. 7. Musch DC, Lichter PR, Guire KE, et al. The Collaborative Initial Glaucoma Treatment Study: study design, methods and baseline characteristics of enrolled patients. Ophthalmology. 1999;106(4):653-62. 8. Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108(11):1943-53. 9. Musch DC, Gillespie BW, Lichter PR, et al. Visual field progression in the Collaborative Initial Glaucoma Treatment Study the impact of treatment and other baseline factors. Ophthalmology. 2009;116(2):200-7. 10. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol.1998;126(4):487-97. 11. Drance S, Anderson DR, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131(6):699-708. 12. Anderson DR, Drance SM, Schulzer M. Natural history of normal-tension glaucoma. Opthalmology. 2001;108(2):247-53. 13. Ederer F, Gassterland DE, Sullivan EK. The Advanced Glaucoma Intervention Study (AGIS): 1. Study design and methods and baseline characteristics of study patients. Control Clin Trials. 1994;15(4):299-325. 14. The Advanced Glaucoma Intervention Study (AGIS): 4. Comparison of treatment outcomes within race. Seven-year results. Ophthalmology. 1998;105(7):1146-64. 15. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429-40. 16. Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema and toxic neuropathy. Arch Ophthalmol. 1982;100(1):135-46. 17. Schuman JS, Hee MR, Puliafito CA, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995;113(5):586-96. 18. Kim NR, Lee ES, Seong GJ, et al. Spectral-domain optical coherence tomography for detection of localized retinal nerve fiber layer defects in patients with open-angle glaucoma. Arch Ophthalmol. 2010;128(9):1121-8. 19. Zhang X, Dastiridou A, Francis BA, et al. Comparison of glaucoma progression detection by optical coherence tomography and visual field. Am J Ophthalmol. 2017;184:63-74. 20. Bhagat P, Deshpande K, Natu B. Utility of ganglion cell complex analysis in early diagnosis and monitoring of glaucoma using a different spectral domain optical coherence tomography. J Curr Glaucoma Practice. 2014;8(3):101-6. 21. Damji KF, Bovell AM, Hodge WG, et al. Selective laser trabeculoplasty versus argon laser trabeculoplasty: results from a 1-year randomised clinical trial. Br J Ophthalmol. 2006;90:1490-4. 22. Richter CU, Shingleton BJ, Bellows AR, et al. Retreatment with argon laser trabeculoplasty. Ophthalmology. 1987;94:1085-9. 23. Hong BK, Winer JC, Martone JF, et al. Repeat selective laser trabeculoplasty. J Glaucoma. 2009;18(3):180-3. 24. Mishima HK, Masuda K, Kitazawa Y, et al. A comparison of latanoprost and timolol in primary open-angle glaucoma and ocular hypertension. A 12-week study. Arch Ophthalmol. 1996;114(8):929-32. 25. Weinreb RN, Ong T, Scassellati Sforzolini B, et al. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open-angle glaucoma: the VOYAGER Study. Br J Ophthalmol. 2015;99(6):738-45. 26. Medeiros FA, Martin KR, Peace J, et al. Comparison of latanoprostene bunod 0.024% and timolol maleate 0.5% in open-angle glaucoma or ocular hypertension: the LUNAR Study. Am J Ophthalmol. 2016;168:250-9. 27. Aerie Pharmaceuticals reports initial Rhopressa phase 3 efficacy results. Aerie Pharmaceuticals. April 23, 2015. investors.aeriepharma.com/news-releases/news-release-details/aerie-pharmaceuticals-reports-initial-rhopressatm-phase-3. Accessed October 15, 2018. 28. Aerie Pharmaceuticals reports positive rocket 4 six-month topline safety and efficacy results for Rhopressa (netarsudil ophthalmic solution) 0.02%. Aerie Pharmaceuticals. April 12, 2017. investors.aeriepharma.com/news-releases/news-release-details/aerie-pharmaceuticals-reports-positive-rocket-4-six-month. Accessed October 15, 2018. 29. Aerie Pharma (AERI) announces statistically significant data from Roclatan phase 3 mercury 1 trial. Street Insider. September 14, 2016. www.streetinsider.com/Corporate+News/Aerie+Pharma+%28AERI%29+Announces+Statistically+Significant+Data+from+Roclatan+Phase+3+Mercury+1+Trial/12032805.html. Accessed October 15, 2018. |

