 |
Over the years, we’ve become increasingly comfortable with the safety and predictable outcomes of modern refractive surgery. Indeed, many of the concerns we once had are less worrisome today because our screening protocols and pre-treatment routines have evolved. This is certainly true of our enhanced capacity to pretreat dry eye and ocular surface disease prior to laser-assisted in situ keratomileusis (LASIK) surgery. Doing so improves outcomes as well as the experience for the patient, the optometrist and the surgeon.
But ocular surface disease isn’t the only thing we need to watch out for when examining refractive surgery candidates. We also need to diligently screen for corneal degenerations and dystrophies. These conditions contraindicate surgery due to the likelihood of progression as in the case of keratoconus or exacerbation as in the case of transforming growth factor beta-induced (TGFB-I) dystrophies. Yet, these can be difficult to identify based on family history and clinical exam alone.1
Fortunately, optometrists have newer tools at our disposal to help identify patients before it’s too late. Furthermore, optometry’s genetic screening strategies have recently evolved from testing five types of genetic mutations that result in corneal dystrophy to 70. This technology, the AvaGen (Avellino) test, identifies whether a patient has the genetic mutation responsible for type II granular corneal dystrophies (GCD2). The test uses a simple buccal or cheek swab that is sent to a laboratory for molecular testing. The results are then sent back to your office within 24 to 48 hours. This swab can establish more than 1,000 variants across 75 genes for keratoconus, allowing us to proactively diagnose corneal disease before symptoms ever appear.
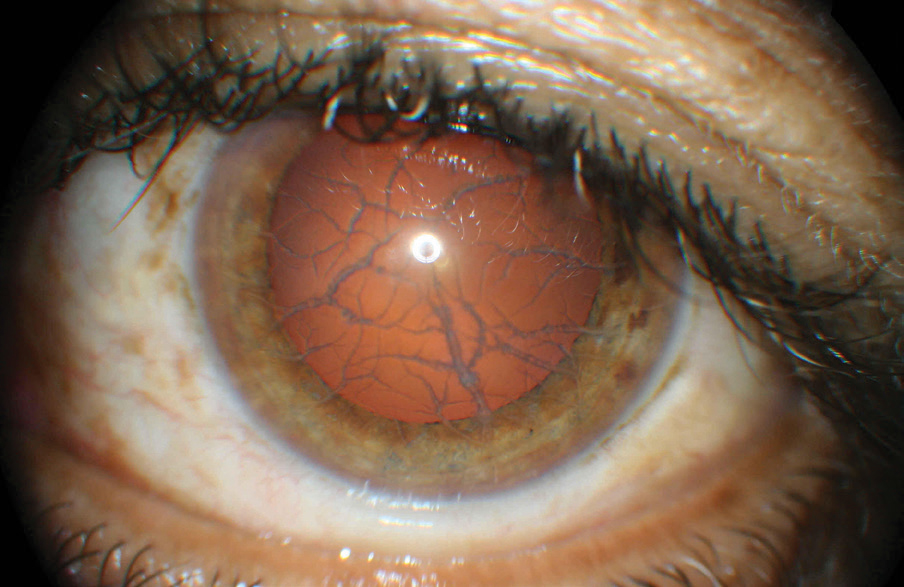 |
| This patient’s anterior segment shows clear signs of lattice dystrophy. This potential consequence of LASIK can be avoided with a proper evaluation that now includes genetic testing. Click image to enlarge. |
Establishing Risk
In determining a patient’s suitability for refractive surgery, AvaGen tests for keratoconus can be extremely useful.
If the swab tests positive, refractive surgery should not be considered, since it could lead to accelerated disease exacerbation characterized by bilateral opacity in the anterior corneal stroma, leading to a severe decrease in best-corrected visual acuity.14
One way to understand who might be at the greatest risk of developing more advanced disease is to understand the natural progression of keratoconus. In a systematic review and meta-analysis of 41 publications reporting various outcomes over 12 months on 11,529 untreated eyes with keratoconus, three factors were found to be statistically significantly associated with the risk of progression.2
The first significant factor was ethnicity.2 When comparing the risk of progression between East Asians, Europeans and Middle Eastern populations, Middle Eastern populations showed a significantly greater risk, followed by Europeans and then East Asians.2
The second factor was age. Younger patients had more aggressive progression and steepening of Kmax at 12 months.2 In fact, the authors predicted that for every 10-year increase in age they’d see 0.8D less Kmax steepening.2 Their models predicted that patients younger than age 17 are likely to have more than 1.5D of Kmax progression at one year.2
Finally, the third factor associated with progression was a high Kmax at baseline.2
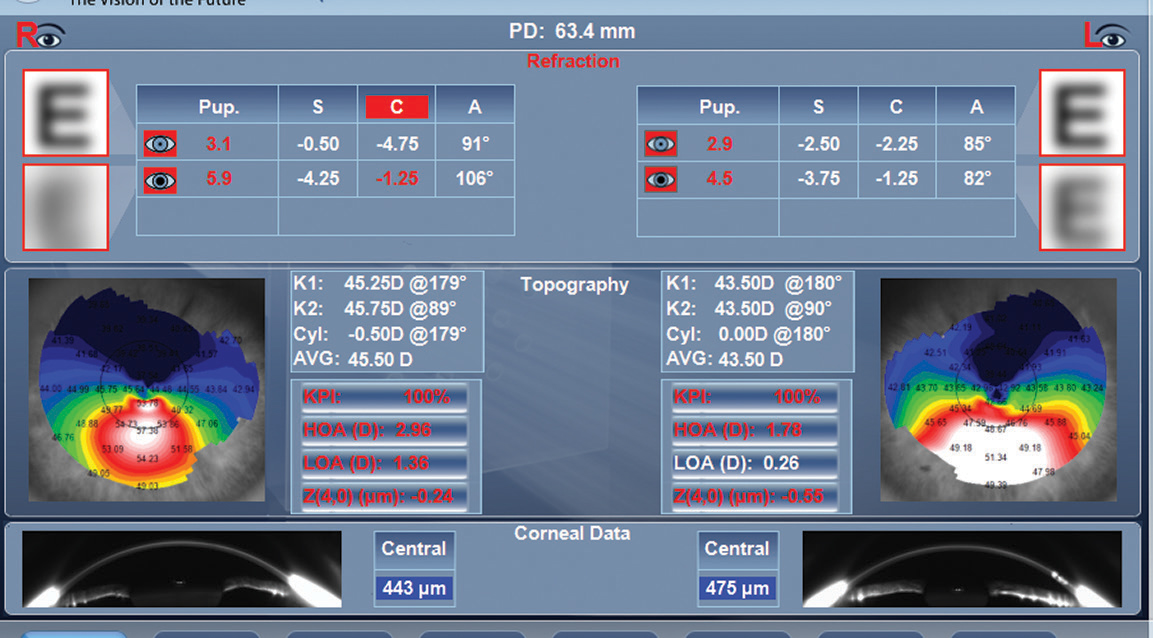 |
| This Luneau VX120 image shows the topography of an eye with keratoconus. Click image to enlarge. |
Granular Corneal Dystrophy
Corneal dystrophies are bilateral, progressive, genetically determined, noninflammatory diseases restricted to the cornea.1
According to the 2008 IC3D classification system, five types of corneal dystrophies are caused by the mutation of TGFB-I gene.3,4 These include lattice corneal dystrophy type I and variants, granular corneal dystrophy type I (GCD1), GCD2 (e.g., Avellino corneal dystrophy), Thiel-Behnke corneal dystrophy and Reis-Bückler corneal dystrophy.3
Initially, researchers knew of no distinction between types I and II GCD.5 However, in 1988 investigators presented the histopathology of corneal buttons from four patients whose origins could be traced to the Italian province Avellino. They each underwent unilateral keratoplasty because of decreased vision.6,7 These patients were clinically diagnosed as GCD2. The cause was found in a mutation of the TGFB-I gene.8,9
In patients with GCD2, vision slowly decreases with age as the central visual axis becomes progressively opaque and painful, recurrent epithelial erosions are common.9,10
GCD2 and Refractive Surgery
Due to the likelihood of recurrence and exacerbation, corneal dystrophies, including GCD2, contraindicate refractive surgery.11 Many cases of post-laser surgery exacerbation have been reported worldwide since GCD2 was first identified.1,3,5,12 But how can we most effectively determine the presence of disease in refractive surgery candidates?
Traditionally, the diagnosis and classification of corneal dystrophies were based on corneal signs and clinical symptoms.13 However, depending on the precise mutation of the disease, patients can be clinically asymptomatic and “dystrophy-free” prior to LASIK, yet they can develop post-op deposition as a result of surgically-induced trauma.1,11 Injury to the central cornea results in exacerbation of the GCD2 with dramatic acceleration of corneal deposition, opacities, and consequent visual loss.3
Because LASIK is strongly contraindicated in GCD2 and surgical candidates with the TGFB-I gene mutations may be asymptomatic, it has been proposed that preoperative genetic screening for TGFB-I mutations should be mandatory for refractive surgery candidates with risk factors.3
Since a lack of clinical signs does not mean the absence of corneal dystrophy-causing mutations and several reports highlight that LASIK treatment induces moderate-to-severe early exacerbations of GCD2 and a marked acceleration in disease course with rapidly worsening visual outcome, genetic testing is a welcome safeguard that can be easily added to a standard examination routine.1,5
Note: Dr. Karpecki consults for companies with products and services relevant to this topic.
| 1. Mazzotta C, Traversi C, Baiocchi S, et al. Phenotypic spectrum of granular corneal dystrophy type II in two Italian families presenting an unusual granular corneal dystrophy type I clinical appearance. Case Rep Ophthalmol Med. 2015;2015:703418 2. Ferdi AC, et al. Keratoconus natural progression: A systematic review and meta-analysis of 11,529 eyes. Ophthalmol. 2019;126(7):935-45. 3.Chao-Shern C, Me R, DeDionisio LA, et al. Post-LASIK exacerbation of granular corneal dystrophy type 2 in members of a chinese family. Eye (Lond). 2018;32(1):39-43. 4. Lee WB, Himmel KS, Hamilton SM, et al. Excimer laser exacerbation of Avellino corneal dystrophy. J Cat Refract Surg. 2007;33(1):133-8. 5. Aldave AJ, Sonmez B, Forstot SL, et al. A clinical and histopathologic examination of accelerated TGFBIp deposition after LASIK in combined granular-lattice corneal dystrophy. Am J Ophthalmol. 2007;143:416-9. 6. Banning CS, Kim WC, Randleman JB, et al. Exacerbation of Avellino corneal dystrophy after LASIK in North America. Cornea 2006;25:482-4. 7. Gruenauer-Kloevekorn C, Braeutigam S, Froster UG, Duncker GI. Surgical outcome after phototherapeutic keratectomy in patients with TGFBI-linked corneal dystrophies in relation to molecular genetic findings. Graefes Arch Clin Exp Ophthalmol. 2009;247:93–9. 8. Jun R, Tchah H, Kim T, et al. Avellino corneal dystrophy after LASIK. Ophthalmol. 2004;111:463-8. 9. Zeng L, Zhao J, Chen Y, et al. TGFBI gene mutation analysis of clinically diagnosed granular corneal dystrophy patients prior to PTK: a pilot study from Eastern China. Sci Rep. 2017;7:596. 10. Park S, Mok J, Joo C, Kim M. Heterozygous avellino corneal dystrophy 9 years after photorefractive keratectomy: natural or laser-induced accelerated course? Cornea. 2009;28(4):465–7. 11. Bower K, Woreta F. Update on contraindications for laser-assisted in situ keratomileusis and photorefractive keratectomy. Curr Opin Ophthalmol. 2014;25(4):251–257. 12. Ellies P, Bejjani RA, Bourges JL, et al. Phototherapeutic keratectomy for BIGH3-linked corneal dystrophy recurring after penetrating keratoplasty. Ophthalmol. 2003; 110: 1119–1125. 13. Inoue T, Watanabe H, Yamamoto S, et al. Recurrence of corneal dystrophy resulting from an R124H Big-h3 mutation after phototherapeutic keratectomy. Cornea. 2002;21(6):570–3. 14. Szentmáry N, Seitz B, Langenbucher A, Schlötzer-Schrehardt U, Hofmann-Rummelt C, Naumann GO. Histologic and ultrastructural changes in corneas with granular and macular dystrophy after excimer laser phototherapeutic keratectomy. Cornea. 2006;25(5):257–63. 15. Awwad ST, Di Pascuale MA, Hogan RN, Forstot SL, McCulley JP, Cavanagh HD. Avellino corneal dystrophy worsening after laser in situ keratomileusis: further clinicopathologic observations and proposed pathogenesis. Am J Ophthalmol. 2008;145(4):656–661. |

