The reason for the outbreak of fungal keratitis cases remains unknown. At press time, the Centers for Disease Control and Prevention reported 102 confirmed cases, 12 possible cases and 81 cases under investigation.
The CDC had complete data on 58 of the confirmed cases, and 56 of these were contact lens wearers. Among these, 54 reported using a Bausch & Lomb ReNu solution, 32 of which used ReNu with MoistureLoc. Some patients used more than one solution, including products from Alcon and AMO.
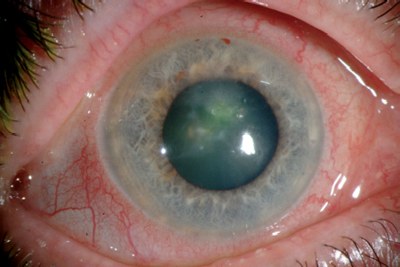 |
| A recent case of fungal keratitis due to infection from fusarium solani. Photo: George O.D. Rosenwasser, M.D. |
Cases of fungal keratitis have been confirmed or are under investigation in 31 states, the CDC says. This follows a similar outbreak in Singapore, where 75 cases have been reported, and in Hong Kong, where 32 cases have been found.
Why is this infection appearing now when generally its most prevalent in warmer climates? asks optometrist Joseph Shovlin, of Scranton, Pa., associate clinical editor of Review of Optometry. Is there some mechanism whereby the normally effective disinfectant is rendered ineffective? And if so, by what mechanism?
Optometrist Brian Levy, chief medical officer at Bausch & Lomb, says, Weve done a lot of work, and at this point we still havent found a cause and effect. Were obviously very concerned because safety is our number one issue. These patients are getting a severe infection, and were still trying to find out why.
In mid-April, B&L pulled ReNu with MoistureLoc from store shelves. Meanwhile, the company has been rigorously testing this product to make sure that it met spec, and that it was killing the bugs and the fungus that its supposed to kill, Dr. Levy says.
Specifically, he says, the company has investigated:
The manufacturing plant. The products in question all seem to have originated at the companys plant in Greenville, S.C. In-house testing there found no contamination in the product or the facility.
Different product lots. The company tested recovered off-the-shelf products from Singapore, Hong Kong and the United States, as well as retained products from the same lots sent to these countries. Chemical testing found that the product met specifications, meaning that the ingredients and their concentrations were at the levels that they were supposed to be. Biocidal testing found that the solution was as effective as it was when approved by the FDA.
Lens cases. The company tested whether the fungus originated in contact lens cases that were contaminated when shipped. However, testing of shipments of cases found no microbial growth.
But what if the plastic in the lens case itself is absorbing or breaking down the solution? We looked at representative cases and found no significant uptake of disinfectant or anything else in the case, Dr. Levy says. So, it doesnt seem to be a reaction between the case and the solution.
Fungus cultures. Contact lens solutions are tested against a standard lab culture that every company uses, Dr. Levy says. This way, every manufacturer tests against the same standard. In addition, B&L obtained a culture from a U.S. patients fusarium infection. When tested against both the lab culture and the wild strain culture, the solution still achieved its known disinfection rate.
The company is also gathering fungal cultures from cases in affected countries. Initially, we did find some differences between some of those [strains] in Asia and some of those in the U.S., but were waiting for the final analysis and genotyping to be completed on all of the isolates, Dr. Levy says.
Used lenses. B&L took contact lenses from people in its clinic, put the used lenses into lens cases and inoculated the lenses with fusarium (obtained both from the lab culture and the wild strain culture). After a four-hour soak in ReNu, the solution was still effective.
Solution components. The company broke down the solution into its individual components to see whether or not fusarium would actually grow on those things instead of remaining dormant, Dr. Levy says. We made 44 formulations from the components and inoculated each with fusarium. We didnt find any growth, and in some cases we found the fusarium to be inhibited.
In 2004, Bausch & Lomb reformulated ReNu Multiplus solution and changed it to ReNu with MoistureLoc. At that time, the company replaced the disinfection agent polyhexamethylene biguanide (PHMB) with another biguanide called Alexidine. The disinfection rates of both agents are about the same, Dr. Levy says, but MoistureLoc was even better against fusarium.
One characteristic that affected patients typically have in common: poor lens care habits.
Thats certainly true in four recent cases seen by ophthalmologist George O.D. Rosenwasser, M.D., of the Central Pennsylvania Eye Institute, in Hershey, Pa. When Dr. Rosenwasser sent one of these patients contact lenses and lens case to be cultured, the microbiologist sent back a report saying the lenses were coated with fusarium and the lens case was teeming with the fungus.
Three of these patients presented in the past month and are still undergoing intense treatment, Dr. Rosenwasser says. The fourth patient, who presented in June 2005, has since had a corneal graft. The patients in the three recent cases used ReNu with MoistureLoc, he says. In the first case, we were not looking specifically for a disinfectant connection.
In terms of treatment, Natacyn (natamycin 5%, Alcon) is the initial choice for many doctors. However, Dr. Rosenwasser says topical voriconizole (compounded from a specialty pharmacy) can be more effective in eradicating the pathogen. He prescribes it hourly during the day, and q2h or even hourly at night, until the cornea is clear. Advanced cases require more aggressive therapy, he says.
In that regard, dont take chances with these patients. If you have any suspicion of fusarium, proceed with caution until proven otherwise, Dr. Rosenwasser says. And certainly dont treat possible fungal keratitis patients with steroids, which will cause the infection to proliferate, he adds.
If you dont have access to appropriate culture mediaIm not talking about a swab here, Im talking about corneal scrapings planted directly to a culture platethen you dont want to mess around with it, he says.
Lawyers have filed lawsuits on behalf of several patients against Bausch & Lomb. In the meantime, B&L is working with the FDA and CDC to determine the source of these infections.

