Annual Cornea ReportFollow the links below to read other articles from our annual Cornea Report: An OD’s Guide to Corneal Transplant Options |
Corneal metabolism and wound healing are crucial to properly maintaining the cornea’s integrity and functionality. When an insult occurs to the corneal surface, creating an epithelial defect, the complex re-epithelialization process involves limbal stem cells, cell differentiation, proliferation, migration and remodeling of the extracellular matrix.1 Researchers believe growth factors involved in the process include epidermal, keratinocyte, hepatocyte and basic fibroblast growth factors.2 In normal, healthy corneas, supportive therapy can help the body resolve an epithelial defect rapidly. However, healing may be delayed or halted altogether in compromised conditions, leaving the underlying stroma exposed and vulnerable to further trauma, infiltrates, infection, scarring or perforation.3 A persistent epithelial defect, defined as a defect that has not resolved after two weeks of standard treatment, is a significant long-term management problem for ODs.4
Some risk factors that can confound corneal epithelial healing include trauma, diabetic keratopathy, limbal stem cell deficiency, dry eye disease (DED), exposure keratopathy, neurotrophic keratopathy after penetrating keratoplasty (PKP)and herpetic infections and diabetic vitrectomy.5 Each condition can affect the normal metabolism of the epithelium and delay, disrupt or suspend healing of an epithelial insult.
The main therapeutic goal is to provide an environment conducive for the eye to restart and complete the epithelialization process. This usually involves providing extra lubrication and supporting the ocular surface to allow for the normal proliferation and migration of differentiated epithelial cells to cover the defect.
Early intervention and resolution is key, as research shows the length of time a defect is left open is proportional to the time it will take for the defect to be fully repaired.6,7
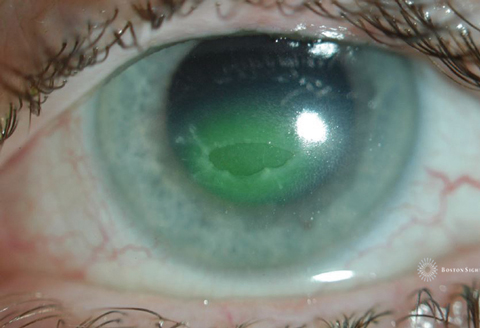 |
| Fig. 1. Diffuse fluorescein staining with white light reveals this epithelial defect. Click image to enlarge. |
Assessment
When evaluating a persistent epithelial defect, clinicians should carefully record both positive and negative pertinent findings. To properly monitor the healing process, clinicians should record the size and location of the defect at each visit and image in white light and blue light immediately after fluorescein instillation (Figures 1 and 2).
Clinical experience suggests the size and shape of the defect is most conspicuous immediately after fluorescein instillation; thus, measurements and photos should be taken right away. Depending on the depth of the defect, a delay of even five minutes may result in blurred margins as the fluorescein absorbs into the surrounding epithelium and underlying stroma—making the margins harder to discern.
In addition, clinicians should monitor for any change in inflammation throughout the management period by noting anterior chamber reaction for inflammatory cells and flare. Underlying or associated haze or infiltrates may be red flags for concurrent infectious activity. If all is quiet, the lack of inflammatory cells and flare can be recorded as pertinent negative findings to confirm there is no concurrent inflammatory or infectious activity.
During treatment, the patient should be evaluated frequently, even daily initially, to monitor progress.
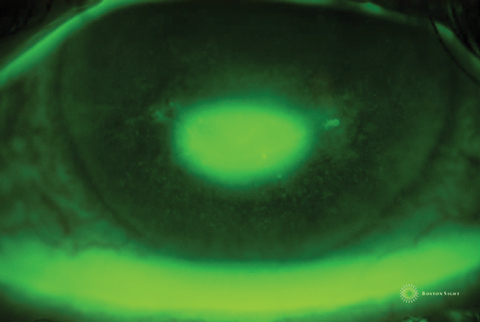 |
| Fig. 2. Picture of an epithelial defect taken several minutes after fluorescein instillation. As the fluorescein absorbs into the stroma, the edges are obscured, making it difficult to determine its exact size and shape. Click image to enlarge. |
Standard Treatments
Many factors will influence your treatment regimen, including concurrent conditions, the patient’s systemic health, medication use and response to treatment. Here is a look at the treatment options available and when it’s best to use them:
Etiologies of Persistent Epithelial Defect3
|
In addition, many concurrent topical medications have known corneal toxicity and can negatively impact the healing process for epithelial defects.9-11 The topical aminoglycosides gentamicin and tobramycin, for example, can cause superficial punctate keratitis in addition to delaying corneal healing.12 Similarly, topical ciprofloxacin and the topical nonsteroidal anti-inflammatory drugs (NSAIDs) diclofenac and ketorolac can also adversely affect corneal wound healing.13,14 In addition, commonly prescribed glaucoma medications such as latanoprost, travoprost, brinzolamide and dorzolamide cause low-grade chronic inflammation with prolonged use.9
Perhaps more importantly, the ubiquitous preservative benzalkonium chloride (BAK) is a well-known ocular surface irritant. The wide use of BAK is due largely to its weak allergenic potential and high rate of antimicrobial properties. However, research demonstrates its toxic effects on the ocular surface, and some studies show significantly fewer symptoms and signs when patients use preservative-free glaucoma medications.15,16
Whenever possible, clinicians should modify a patient’s medication use during the treatment period to decrease the effect of medicamentosa and provide an environment more conducive to corneal healing.
Aggressive lubrication. Bathing the cornea in adequate lubrication should be the first line of attack to initiate the epithelialization process. Depending on the underlying cause of the persistent defect, poor surface lubricity could be the main reason for delayed resolution. Ointment or preservative-free artificial tears should be generously applied every hour for ointment, every half hour with artificial tears. The adage “you can’t use too much” certainly applies here. Ointment, though it is accompanied by concomitant visual symptoms, is the preferred modality because of the increased contact time with the cornea.3
Punctal occlusion. Another measure to provide a more lubricious environment for the ocular surface is punctal occlusion in the presence of dry eye syndrome. However, punctal occlusion may exacerbate any toxicity present from topical medications, and clinicians should be aware of all medications the patient is using to avoid adverse effects.3
Bandage soft contact lenses. The use of a silicone hydrogel contact lens can be effective in protecting the underlying cornea from the shearing forces of the lid that occur with every blink. Such protection is particularly helpful for newly formed cells attempting to migrate and form a new epithelial layer. Because of the risk of infectious keratitis, these patients should be seen frequently to monitor the healing process and rule out infection. Concurrent topical preservative-free antibiotics should be prescribed for prophylaxis.
Pressure patching. Though a common treatment option in the past, evidence suggests pressure patching can actually impede the healing process and be a source of infection.17-20 Several studies show either no additional benefit or delayed healing with pressure patching compared with use of antibiotic and mydriatic alone.19,21
 |
| Fig. 3. This patient has a history of neurotrophic keratopathy secondary to herpes simplex keratitis. He suffered multiple episodes of surface breakdown that ultimately responded to a lateral tarsorrhaphy, which has been kept in place for surface maintenance. |
Secondary Options
In spite of many traditional treatment options, some conditions may prove to be recalcitrant and require secondary therapies:
Scleral lenses. Research suggests scleral lenses and PROSE (for “prosthetic replacement of the ocular surface ecosystem”) devices (BostonSight) can be effective in the treatment and resolution of epithelial defects.22-25 PROSE is a medical treatment model developed to treat complex corneal conditions. Treatment involves the customized fitting of ocular prosthetic devices to achieve one or more of the following goals: improve vision (particularly for irregular corneas), improve comfort (for severe ocular surface disease) and support the ocular surface (for pathology of ocular surface disease). For persistent epithelial defects, the device may help to provide an environment that is conducive to corneal surface rehabilitation by continually bathing the ocular surface and providing a mechanical barrier against the eyelid during blink related micro-trauma.22,25
The treatment of a persistent epithelial defect with scleral lenses or PROSE devices involves overnight wear with daily monitoring to track resolution of the defect and check for any infectious or inflammatory events. Daily wear will provide only a partial benefit, as healing improves much quicker with overnight wear. A drop of preservative-free moxifloxacin is applied, either in the eye prior to lens insertion or into the reservoir with preservative-free saline. Vault should be sufficient to clear the limbus. Based on clinical experience, the key is to fit the lens as loose as possible to reduce suction and inflammatory triggers that come from a tight-fitting lens. Typically, a larger diameter lens with adequate toricity will work better for overnight wear than a smaller lens that may have more suction. If the patient can tolerate the awareness that comes with edge lift of the peripheral curve, this would be acceptable, even preferred, to create a looser fitting lens.
Anecdotally, though longstanding persistent epithelial defects may not respond to one night of scleral lens wear, once healing is initiated after several nights, the healing rate will increase and full resolution will occur soon thereafter.
After resolution of the defect, researchers postulate that continuing overnight wear for 24 to 48 hours may reduce recurrence of surface breakdown.
Therapies in the PipelineOther treatments in development are promising: Matrix regenerating agent. Recently available in Europe, this is a large biopolymer that is an analog of glycosaminoglycan integral to the structure of the extracellular matrix. Research demonstrates topical application effectively heals persistent epithelial defects after fortified antibiotic treatment of bacterial keratitis.37 Amniotic membrane extract. Research is being conducted on a lyophilized preparation of amniotic membrane that can be used as an eye drop.2,36 The presence of growth factors in this extract could potentially have similar efficacy in healing of amniotic membrane in topical form the patient applies at home without the need for in office-application. |
Amniotic membrane grafting. Since their introduction in 1995, amniotic membrane grafts have been used for many ocular surface conditions, including persistent epithelial defects.26,27 Two types are available: cryo-preserved and epithelialized. The latter of the two needs to be maintained at -80°C and dehydrated, unless it is de-epithelialized, in which case it can be stored at room temperature.26 Prokera (Bio-Tissue) cryopreserved amniotic membrane is a commonly-used option that can be inserted in-office with relative ease, though some patients find the ring to be a source of discomfort. BioDOptix (BioD) and AmbioDisk (Katena) are other amniotic membrane options.
The role of amniotic grafts in re-epithelialization of persistent epithelial defects may involve a mechanical effect where the basement membrane of the graft serves as a scaffolding on which regenerating epithelial cells can migrate.26 Researchers speculate that the basement membrane also reinforces adhesion of basal epithelial cells and promotes epithelial differentiation.28,29 In addition, the growth factors necessary for healing are present in amniotic membrane.30
Autologous serum. This can be beneficial to initiating and expediting the healing of an epithelial defect because it contains the growth factors necessary for re-epithelialization.7,31,32 Clinicians often advocate for frequent instillation, such as one drop every two hours, to expedite healing. One study found an average healing time of 22 days with the use of 50% serum drops.7
Recent evidence suggests the use of autologous serum eye drops with silicone hydrogel soft contact lenses is an effective combination therapy for persistent epithelial defects.33-35 This protocol combines the mechanical protection of a bandage soft contact lens with the nutrients of autologous serum eye drops to provide a synergistic effect on healing. The use of autologous serum after resolution of the epithelial defect with a bandage contact lens also showed less recurrence.33
However, logistical obstacles to using autologous serum may limit its utility. The process of attaining the drops first requires a blood draw, which may be prohibitive for patients with limited access to care or who are disabled.
Limbal stem cell transplantation. This is an advanced option when other standard and secondary therapies fail for patients with limbal stem cell deficiency as a complicating factor. The procedure involves the transplantation of limbal stem cells to the affected eye.36 The source of the grafted tissue can be either an autograft from the contralateral eye or allograft from a donor. Autologous transplantation of tissue from the contralateral eye will ensure no graft rejection, although the grafted eye may be susceptible to limbal stem cell deficiency.3
Tarsorrhaphy. When other options are unsuccessful or are unavailable, temporary partial or complete tarsorrhaphies can be effective in providing an environment conducive to healing (Figure 3). This is a good option when exposure keratopathy is either contributory to the formation of the defect or may complicate healing. Clinicians should also consider this treatment modality for patients who are noncompliant or are physically unable to apply lubrication drops.3
Despite myraid treatment options, persistent epithelial defects remain challenging entities for even the most seasoned clinician, and patience is a key virtue. Clinicians must be vigilant with follow up and see the patient daily or every other day until resolution, and the timeline depends on the obstinacy of the disease and the effectiveness of the treatment. Some defects may respond fairly quickly (i.e., one to two days with overnight scleral lens wear) or may be more prolonged over several weeks. Once healed, clinicians should ensure patients have sufficient lubrication and manage medicamentosa to reduce the likelihood of recurrence. In most of these vulnerable patients, recurrence of surface breakdown may be common and warrants more frequent examinations beyond the annual visits.
Case ExampleA 38-year-old Caucasian female presented with a history of systemic lupus erythematosus and Stevens-Johnson syndrome with severe ocular complications. She suffered an ulcerative keratitis in the left eye that perforated, requiring a therapeutic PKP. Postoperative assessments showed incomplete re-epithelialization of donor graft that, over a course of a month, did not respond to bandage contact lens treatment and topical gentamicin. She was referred for PROSE treatment and resolution of persistent epithelial defect. Her entering visual acuity with correction (VAcc) was 20/30 OD, 20/400 OS. Anterior segment evaluation was significant for cauterized lower puncta OD, OS, conjunctival injection 3+ temporally and inferiorly OS, a decentered 6mm corneal graft with several intact sutures and active corneal neovascularization to the graft 360 degrees with corneal vessels at the graft-host interface infero-nasal (Figure 1). There was a 3mm epithelial defect 10 to 12 o’clock within graft (Figure 2). The PROSE treatment was initiated OS with the following device parameters: Boston XO2 (Dk: 140), 8.5mm BC, +3.50D, 19.0mm diameter and with-the-rule toric peripheral curves.
The initial device assessment showed alignment of the peripheral haptics. Prior to insertion, a drop of Vigamox was instilled into the reservoir of the device, along with preservative-free saline solution, for prophylaxis against infectious keratitis. The patient was instructed to not remove the device overnight and to return in the morning for evaluation. Follow UpThe next day, the patient’s VAcc was 20/30 OD, 20/125 OS. The anterior segment evaluation showed slightly reduced conjunctival injection at 2+ OS and a significantly reduced epithelial defect on the graft (Figure 3). By the next day after another night of overnight wear, the defect was resolved (Figure 4). Overnight wear of the device was discontinued and erythromycin ointment was prescribed prior to bedtime for overnight lubrication. The patient continues to wear the PROSE device on a daily basis and has had no recurrence of epithelial breakdown. At a recent visit, the eye was white and quiet with ghosted corneal vessels that were previously active (Figure 5). | ||||||||||
Dr. Kwok is a clinician at BostonSight and was previously a faculty member of the New England College of Optometry specializing in contact lens education. He is a graduate of the University of Waterloo School of Optometry and completed a primary care residency at the New England College of Optometry.
1. Ljublimov AV, Saghiizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015;49:17-45. |






